-
PDF
- Split View
-
Views
-
Cite
Cite
Ranamalie Amarasinghe, Jacqueline Poldy, Yuki Matsuba, Russell A. Barrow, Jan M. Hemmi, Eran Pichersky, Rod Peakall, UV-B light contributes directly to the synthesis of chiloglottone floral volatiles, Annals of Botany, Volume 115, Issue 4, March 2015, Pages 693–703, https://doi.org/10.1093/aob/mcu262
Close - Share Icon Share
Abstract
Background and Aims Australian sexually deceptive Chiloglottis orchids attract their specific male wasp pollinators by means of 2,5-dialkylcyclohexane-1,3-diones or ‘chiloglottones’, representing a newly discovered class of volatiles with unique structures. This study investigated the hypothesis that UV-B light at low intensities is directly required for chiloglottone biosynthesis in Chiloglottis trapeziformis.
Methods Chiloglottone production occurs only in specific tissue (the callus) of the labellum. Cut buds and flowers, and whole plants with buds and flowers, sourced from the field, were kept in a growth chamber and interactions between growth stage of the flowers and duration and intensity of UV-B exposure on chiloglottone production were studied. The effects of the protein synthesis inhibitor cycloheximide were also examined.
Key Results Chiloglottone was not present in buds, but was detected in buds that were manually opened and then exposed to sunlight, or artificial UV-B light for ≥5 min. Spectrophotometry revealed that the sepals and petals blocked UV-B light from reaching the labellum inside the bud. Rates of chiloglottone production increased with developmental stage, increasing exposure time and increasing UV-B irradiance intensity. Cycloheximide did not inhibit the initial production of chiloglottone within 5 min of UV-B exposure. However, inhibition of chiloglottone production by cycloheximide occurred over 2 h of UV-B exposure, indicating a requirement for de novo protein synthesis to sustain chiloglottone production under UV-B.
Conclusions The sepals and petals of Chiloglottis orchids strongly block UV-B wavelengths of light, preventing chiloglottone production inside the bud. While initiation of chiloglottone biosynthesis requires only UV-B light, sustained chiloglottone biosynthesis requires both UV-B and de novo protein biosynthesis. The internal amounts of chiloglottone in a flower reflect the interplay between developmental stage, duration and intensity of UV-B exposure, de novo protein synthesis, and feedback loops linked to the starting amount of chiloglottone. It is concluded that UV-B light contributes directly to chiloglottone biosynthesis. These findings suggest an entirely new and unexpected biochemical reaction that might also occur in taxa other than these orchids.
INTRODUCTION
Plants use a diverse range of visual and olfactory cues to attract animal pollinators (Raguso, 2004; Whitehead et al., 2012), with floral volatiles often particularly critical for securing insect pollination (Pichersky and Gershenzon, 2002; Raguso, 2008; Muhlemann et al., 2014). More than 1700 different compounds have been documented in plant floral odours (Knudsen et al., 1993, 2006); however, relatively few studies have determined the precise role of these compounds in pollinator attraction (Bohman et al., 2014). There are even fewer cases where we have a comprehensive understanding of the physiology, biochemistry and biosynthesis of the floral volatile compounds involved in pollination.
The sexually deceptive orchids are a group of plants for which specific semiochemicals play a key role in pollination. These orchids lure specific male insect pollinators to their flower by emitting volatile semiochemicals that mimic the female sex pheromone (Peakall, 1990; Schiestl et al., 2003; Franke et al., 2009; Ayasse et al., 2011). Remarkably, this pollination strategy has evolved independently in orchids on at least four continents (Africa, Australia, Europe and South America) (Gaskett, 2011), and new discoveries continue to be made within this family (Phillips et al., 2014). This pollination strategy has also recently been discovered in the bee-fly-pollinated Gorteria diffusa (Asteraceae) (Ellis and Johnson, 2010; de Jager and Ellis, 2012) and in the bee-pollinated Iris paradoxa (Iridaceae) (Vereecken et al., 2012).
Australia is a world centre of diversity for the pollination strategy of sexual deception, with several hundred terrestrial orchid species across at least ten genera involved. These orchids sexually exploit male insects from five different groups of Hymenoptera (ichneumonid, thynnine and scoliid wasps, ants and saw flies) and the Diptera (fungus gnats) (Peakall, 1990; Gaskett, 2011; Phillips et al., 2014). In this study, we focus on the orchid genus Chiloglottis, representing the largest exclusively sexually deceptive genus in Australia, with some 30 species, although the taxonomy is not fully resolved.
The specific interaction between Australian Chiloglottis orchids and their male thynnine wasp pollinators involves 2,5-dialkylcyclohexane-1,3-diones or ‘chiloglottones’ (Schiestl et al., 2003; Poldy et al., 2008, 2009, 2012; Franke et al., 2009; Peakall et al., 2010). Chiloglottones have also been found in Arthrochilus and Paracaleana, two related sexually deceptive genera where they are also implicated in pollinator attraction (Peakall et al., 2010; Bohman et al., 2014). However, floral volatile surveys of six other Australian sexually deceptive genera (Caleana, Caladenia, Cryptostylis, Drakaea, Leporella and Spiculaea) have not detected chiloglottones (see Bohman et al., 2014). In Drakaea, the putative sister genus to Chiloglottis, a specific blend of alkypyrazines and one hydroxymethyl pyrazine is used to secure male thynnine wasp pollination in D. glyptodon (Bohman et al., 2014). Pyrazines are probably involved in pollination of other Drakaea and some Caladenia (Bohman et al., 2012a, b, 2013), but they have not been found in other sexually deceptive orchid genera surveyed (Bohman et al., 2014). Thus, across sexually deceptive Australian orchids, several different classes of volatile semiochemicals are expected to be involved in pollinator attraction.
While chiloglottones may be seen as additional example of the already large number of plant volatiles known (Dudareva and Pichersky, 2000; Pichersky and Gang, 2000; Pichersky et al., 2006; Pichersky and Lewinsohn, 2011; Muhlemann et al., 2014), their unique structures have not been detected before in plants or elsewhere (but are now known to be present in the wasp pollinators as well). Thus, as a totally new class of volatiles (Franke et al., 2009), their mode of synthesis in the plant is presently unknown. Much progress has been made towards understanding how sexual deception is achieved in Chiloglottis, including the elucidation of the unique chemistry across multiple orchid–wasp interactions, discovery of the mechanisms controlling pollinator specificity and identification of a key role for chemistry in speciation (Peakall et al., 2010; Peakall and Whitehead, 2014; Whitehead and Peakall, 2014). In contrast, research on the physiology, biochemistry and molecular biology of chiloglottone production is only now beginning. Nevertheless, this research is already indicating new and unexpected discoveries.
Initial physiological investigations have focused on two Chiloglottis taxa representing different clades, C. trapeziformis and C. seminuda (Falara et al., 2013). Both species employ chiloglottone 1 (2-ethyl-5-propylcyclohexane-1,3-dione) to attract their respective specific male thynnine wasp pollinators (Peakall et al., 2010; Griffiths et al., 2011). This semiochemical can be readily detected by solvent extraction of flowers. However, chiloglottone cannot be detected by headspace or solid phase microextraction (SPME) techniques (Falara et al., 2013). Thus, it is presently only possible to quantify the internal amount of the metabolite (ng per flower), not odour emission. Average chiloglottone 1 amounts are highest in mature flowers (>4 d old) and show no diurnal fluctuations (Falara et al., 2013). Chiloglottone 1 has not been detected in buds of either species, consistent with the observations in many other plants that flowers only begin to synthesize and emit scent after flower opening (Pichersky et al., 1994; Dudareva and Pichersky, 2000; Piechulla and Effmert, 2010).
Chiloglottone production is tissue specific and differs between the two species. In C. trapeziformis, chiloglottone 1 is produced only by the densely clustered calli (callus) that are attached to the labellum by a stalk. In C. seminuda, the calli are more extensively developed, extending across the posterior two-thirds of the labellum to form a structure that approximates the shape and size of the female thynnine wasp [for details, see fig. 1 in Falara et al. (2013)]. Chiloglottone 1 production occurs in this callus region of the labellum, which cannot be separated from the labellum lamina, as well as in the two lateral sepals (but not the dorsal sepal).
Observations that plants grown in growth chambers under visible light, but lacking light in the range <400 nm, flowered, but did not produce chiloglottone 1, suggested that production of this volatile might require UV light. Furthermore, in both C. trapeziformis and C. seminuda, flowers collected from the field (and containing chiloglottone 1 at collection) become depleted of chiloglottone over several days in a growth chamber lacking UV light. Upon re-exposure to sunlight, chiloglottone 1 becomes detectable in just 2 min, with quantifiable amounts (ng per callus) of chiloglottone detected within 15 min, and normal amounts found within 2–3 h. Further experiments revealed that maximum chiloglottone 1 amounts were obtained when flowers were exposed to light in the UV-B range (280–315 nm) (Falara et al., 2013). The possibility that UV-B light at the naturally low intensities found in normal sunlight is required for the biochemical reaction itself is new and unexpected, and might occur in taxa other than these orchids.
As a result of declines in atmospheric ozone that have heightened concerns about the future impacts of increased UV radiation on ecological systems, and its interplay with climate change, most UV-related research has focused on the potential negative consequences of high UV radiation intensity (e.g. Frohnmeyer and Staiger, 2003; Arróniz-Crespo et al., 2011; Ballare et al., 2011, but see Ioannidis et al., 2002; Zhang and Bjorn, 2009 for exceptions). Excess UV-B radiation is well known to influence the accumulation of phenolics, carotenoids and glucosinolates in plants, with these compounds collectively providing antioxidant and UV screening functions (Zhang and Bjorn, 2009; Schreiner et al., 2012). However, presently there appears to be little evidence that UV-B radiation directly influences the production of plant floral volatiles. In sweet basil (Ocimum basilicum), experiments with supplementary UV-B radiation indicate that UV-B is required for the normal development of oil glands, but not the production of the volatiles that fill the glands (Ioannidis et al., 2002). With the recent molecular and chemical characterization of the first UV-B photoreceptor, UVR8 (Rizzini et al., 2011; Heijde and Ulm, 2012; Voityuk et al., 2014), there is now growing interest in UV-B-specific regulatory events that are triggered by low, naturally occurring, doses of UV-B (Đinh et al., 2013; Morales et al., 2013; Jenkins, 2014; Robson et al., 2014). Beyond UVR8, it is considered possible that other yet to be discovered UV-B photoreceptors may exist in plants (Jenkins, 2014). Thus, there is still much to learn about the interactions between UV-B and plant physiology, biochemistry and biosynthesis.
In Chiloglottis trapeziformis and C. seminuda, the rapid production of detectable chiloglottone 1 within minutes of UV-B exposure in depleted flowers may suggest (Falara et al., 2013) that the enzymes and substrates directly involved in biosynthesis are already present in the tissue, and that UV-B light is required for the reaction itself. However, a requirement for de novo synthesis of proteins to reach the maximum chiloglottone amounts is also suggested by the increase in chiloglottone production with time. The observation that chiloglottone has not been found in buds has now allowed us to address the following questions that arise from the above observations. (1) Does the enclosure of the specific chiloglottone-producing tissue(s) within the bud block the entry of UV-B? (2) Can manually opened buds produce chiloglottone upon UV-B exposure? (3) Does blocking protein synthesis affect chiloglottone biosynthesis?
MATERIALS AND METHODS
Floral material
Flowers and buds of Chiloglottis trapeziformis were obtained from a colony growing naturally within the Australian National Botanic Gardens (Canberra, ACT, Australia), with samples taken regularly across the flowering season (August to November) in 2012 and 2013. Chiloglottis valida was sourced from wild populations in the Kosciuszko National Park (NSW, Australia) in November 2012. Chiloglottis seminuda flowers were obtained from wild populations in the vicinity of Mt. Werong in the Blue Mountains (NSW, Australia) in March 2013.
Plant growth conditions
Both cut buds and flowers, and whole plants with buds and flowers, sourced from the field, were kept in a growth chamber prior to and during experiments under the following conditions: a photoperiod of 12/12 h day/night; light intensity of 300 µmol m−2 s−1 of white light lacking UV light <400 nm; and temperature and relative humidity of 20/15 °C and 79 %/84 %, respectively, during day/night.
Preparation of buds and flowers for experiments
Buds and flowers of C. trapeziformis can be separated into four recognizable phases. Young buds, mature buds, freshly opened flowers (1–2 d after opening) characterized by all green flower parts, and mature flowers (>3 d) characterized by reddish green flower parts (Falara et al., 2013). In this study, the bud classification was expanded to four stages: (1) very young buds (vyb; where both the stalk and the buds were green); (2) young buds (yb; where buds were green but the stalk had developed purple coloration, more mature than very young buds); (3) mature buds (mb; where both bud and stalk were purple, but petals and sepals remained tightly closed); and (4) very mature buds (vmb; where sepals had begun to separate from petals and buds were about to open) according to their appearance (see Supplementary Data Table S1 for expanded details and Fig. S1 for a photograph of these stages).
Different development stages and different pre-treatments of bud and flowers were applied across the study. Here it is important to note that flowers sourced from the field (containing chiloglottone 1) become depleted of chiloglottone over 2–7 d, depending on the starting amount in the flower. For the majority of experiments, it was important to ensure that flowers were fully depleted prior to use. Therefore, sub-samples from each batch of flowers of known collection date were progressively sampled and the amount of chiloglottone 1 per callus measured. Batches of flowers were only considered ‘depleted’ when their relevant sub-sample contained little or no chiloglottone. Buds that opened into flowers in the growth cabinet were also used in some experiments, providing two different treatments: (1) flowers that had never produced chiloglottone [non-sunlight flowers (nsf) with zero chiloglottone 1]; and (2) flowers that had opened during growth which were then exposed to the sun for 6 h and then returned to the growth cabinet for depletion [depleted flower (gdf) with zero chiloglottone 1 initially opening in the growth cabinet]. Buds, whether sourced from the field or developing within the growth chamber (closed), do not contain chiloglottone 1. Therefore, for the experiments investigating UV-B-induced chiloglottone production at different bud developmental stages, it was necessary to open the bud manually by gently loosening the sepals, petals and labellum with forceps to ensure full light could reach all the internal flower parts.
Extraction and quantitation of chiloglottone
Following Falara et al. (2013), the floral parts of interest were dissected from buds and flowers and washed for 3 min in 100 µL of HPLC grade dichloromethane (DCM) containing 20 ng µL−1 of 5-methyl-1,3-cyclohexanedione (Aldrich CAS 4341-24-6) as an internal standard.
Gas chromatographic analysis with mass spectrometry (GC-MS) of the floral tissue extracts was performed on an Agilent Technologies 6890N GC coupled with a 5973 Mass Selective Detector (Agilent Technologies, USA) equipped with a SGE BP21 column (30 m × 0·25 mm × 0·25 µm) connected directly to the MS detector. For each sample, 4 µL of the solvent extract was injected splitless into the inlet at 250 °C, the column was held at 40 °C for 1 min, then programmed at 10 °C min−1 to 230 °C and held for 15 min. Helium was the carrier gas at a flow rate of 2 mL min−1. Quantitation based on corrected percentage areas was performed relative to the internal standard, using the Agilent Technologies Chemstation software.
UV-B treatments in the light box experiments
An in-house custom-made light box (57 × 52 × 42 cm) was used to provide UV-B treatments to cut flowers and buds. Irradiation from above was achieved with UV-B globes from Vilber Lourmat T-15M 15W Lamps (Vilber Lourmat, Germany) and G8T5E Sankyo Denki 8W lamps (Hitachi, Japan). An in-built fan was used to ventilate the chamber (see Supplementary Data Fig. S4 for irradiance spectra of the light sources.)
Spectral transmission measurements through floral parts
Both excised sepals and petals of Chiloglottis buds appear translucent when held in front of a light source, indicating that some visible light can pass through the tissue. Therefore, we employed transmission spectrophotometry to measure the relative percentage of light that is transmitted at different wavelength through the petal into the closed bud. Given that chiloglottone 1 has not been found in buds (Falara et al., 2013), any absorption of UV-B light by the floral tissues could provide mechanical regulation of chiloglottone production.
Transmission was measured with a UV/VIS pre-configured USB2000 portable spectrophotometer (Ocean Optics, FL, USA). Light was from an HPX 2000 UV/VIS light source (Ocean Optics) via a 600 µm optical fibre (UV-VIS) terminating in a 1/16 inch stainless steel ferrule. The end of the fibre was placed almost touching the floral tissue. Transmitted light was collected with a 400 µm fibre (UV-VIS with 1/16 inch stainless steel ferrule) 1 mm from the floral tissue and measured by the spectrophotometer. The two optical fibres were perfectly aligned along the same axis with a custom-machined holder that allowed the two fibres to be moved towards each other without losing alignment. The tissue to be measured was laid flat on a steel aperture with a 1 mm diameter, preventing any stray light impinging on the collecting fibre. All measurements were taken in the dark. An 11 point running average was applied to the calculated transmission data.
Irradiance measurements of UV-B light sources and natural sunlight
Irradiance measurements of the UV-B light sources used in the experiments and irradiance measurements in the field were taken with a UV/VIS pre-configured JAZ-EL200 portable spectrophotometer (Ocean Optics). One end of a 400 µm fibre (QP400-1-UV-VIS) was attached to the spectrophotometer. At the other end, a Cosine Corrector (CC-3-UV-S) was attached for taking light readings sampled across 180 °. Just prior to use, the spectrophotometer with attached fibre and probe was calibrated for absolute irradiance measurements against a radiometrically calibrated DH-2000 deuterium and tungsten halogen light source (220–1050 nm; Ocean Optics), following the manufacturer’s instructions.
Stage dependence and time dependence experiments
The chiloglottone-producing callus tissue on the labellum is entirely hidden within the bud, and chiloglottone 1 has not previously been detected in buds (Falara et al., 2013). However, to investigate if chiloglottone production can occur in manually opened buds, we exposed the labellum of the four different bud stages to sunlight for 2 h after manual opening, and subsequently measured the amount of chiloglottone 1 (ng per callus) in the flowers.
Chiloglottone can be detected within 2 min after exposure to sunlight, with the amount of chiloglottone 1 (ng per callus) known to increase gradually to reach a plateau after 2 h (Falara et al., 2013). Chiloglottone production (ng per callus) was compared over a 4 h time frame among manually opened buds of the four stages [manually opened (mo)-vyb, mo-yb, mo-mb and mo-vmb] and two flower treatments (nsf and gdf). This experiment was conducted over a single day to minimize experimental variation, with sampling at 2, 5, 10, 15, 30, 60, 180 and 240 min.
In all experiments, chiloglottone 1 amounts (ng per callus) were determined for a minimum of three flower replicates (range 3–12) per sampling point, per experiment.
Chiloglottone 1 production and increasing UV-B irradiance
To investigate whether chiloglottone production depends on UV-B radiation intensity, experiments were conducted at four UV-B radiation intensities: 0, 13, 28 and 51 µW cm−2 nm−1 (at 300 nm), representing the absolute irradiance measured for three UV-B lamp configurations (8, 16 and 30 W; see Supplementary Data Fig. S2 for irradiance spectra) and control (covered flowers within light box). This experiment was conducted over a single day to minimize experimental variation and included mo-mb, mo-vmb and depleted flowers initially sourced from the field (fdf) and confirmed to have approximately zero chiloglottone 1 (based on sub-sampling prior to the experiment).
The relationship between chiloglottone 1 production and starting amount
To explore further the interplay between the amount of internal chiloglottone 1 (ng per callus) and induced chiloglottone production, we conducted an experiment in which 50 C. trapeziformis flowers that had opened in the field (and so had produced chiloglottone 1) were harvested and placed in the growth cabinet for depletion. The amounts of chiloglottone 1 in these flowers were determined with either a zero or a 2 h UV-B treatment on days 1, 3, 5 and 7, sacrificing ten flowers at a time.
Protein synthesis inhibition studies
To determine whether or not chiloglottone production requires de novo protein synthesis we employed the de novo protein synthesis inhibitor, cycloheximide (CHX). A CHX (Sigma, USA) stock solution (100 mm) was made with 70 % (v/v) ethanol, and the required amounts were directly added to the final incubation solution to achieve a concentration of 100 µm CHX. Incubations were for the specified periods indicated in the figure legends, prior to 2 h UV-B exposure. The controls consisted of water containing 0·07 % (v/v) ethanol, but no CHX.
Two types of CHX delivery to the target floral tissues were used. Excised callus tissue was incubated for 1 h in 100 µm CHX in water containing 0·07 % (v/v) ethanol prior to 2 h UV-B exposure. Alternatively, CHX was delivered via the stalks of cut buds or flowers held in 5 mL tubes containing the inhibitor solution diluted in 3 mL of water (three buds or flowers per tube). To minimize evaporation of the inhibitor solution, the tube was sealed with parafilm (through which the stalks were inserted). Buds and flowers were retained in this way for both the incubation and the UV-B exposure phases of the experiments.
We initially conducted two parallel experiments using excised callus tissue sourced from mo-vmb vs. from fdf, respectively. In each experiment, we applied two treatments, one with and one without vacuum infiltration, to determine whether the delivery of CHX to the target tissue can be improved by the vacuum infiltration treatment.
Working with excised callus tissue proved difficult for investigating responses to longer CHX incubation times since the excised tissue lost viability and dehydrated under UV-B exposure. Therefore, we used the cut flowers to investigate the changes in chiloglottone 1 after 3, 6, 24 and 72 h of incubation, followed by a 2 h UV-B exposure.
To investigate whether or not de novo protein synthesis is required in the first 2–5 min of chiloglottone production following UV-B induction, mo-mb, mo-vmb and fdf were incubated with CHX for 72 h. The amount of chiloglottone 1 (ng per callus) was then compared across the three floral developmental stages for one set of samples exposed to 5 min UV-B and a second set exposed to UV-B for 2 h.
To evaluate whether or not the response of other Chiloglottis species to CHX and subsequent UV-B exposure is similar to that of C. trapeziformis, we conducted two smaller scale experiments involving C. seminuda and C. valida. Collectively, these three species each represent one of the three major phylogenetic sub-divisions of the genus (Peakall et al., 2010). All three species also use chiloglottone 1 for sexual attraction of their own specific pollinators. Chiloglottis seminuda is of further interest because chiloglottone production occurs in both the calli of the labellum (as for the other two species) and in the distal end of the two lateral sepals (Falara et al., 2013).
Statistical analysis
All statistical analyses were performed in JMP® 9 (SAS Institute). Single-factor analysis of variance (ANOVA) was performed with a priori comparisons among means assessed by the Tukey–Kramer HSD (honest significant difference) test. Linear regression analysis was based on individual sample values grouped as appropriate for the experiment. Following initial graphical inspection of the data, the suitability of linear, two-degree polynomial and log-transformed regression models was determined. Model selection was based on consideration of the probability values and the R2 and root mean square values (RMSE), with the best-fit model characterized by highly significant terms (lowest P-values), the highest R2 values and lowest RMSE. The number of replicates, sample sizes and model outcomes are provided in the respective figure captions. Error bars in the ANOVA figures represent the s.e.m. based on a pooled estimate of error variance, with labelled columns not connected by the same letter deemed significantly different at P < 0·05.
RESULTS
Patterns of light transmission through outer bud tissues
Measurement of the light transmitted from the outside to the inside of the C. trapeziformis petal at the bud stage confirmed that much of the visible light was transmitted through the tissue (Fig. 1). However, UV transmission was negligible below 350 nm.
A graph showing the wavelengths of light transmitted across the UV and visible range (200–850 nm) through the petal tissue (from outside to the inside) of a Chiloglottis trapeziformis bud.
Chiloglottone1 production in manually opened buds
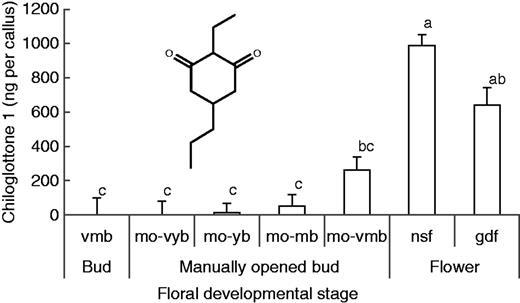
As expected (Falara et al. 2013), no chiloglottone 1 was detected in very mature buds (vmb = naturally closed) after 2 h of exposure to sunlight. However, when buds were manually opened to allow exposure of the labellum to sunlight, chiloglottone was detected in the labellum callus in all but the very young bud stage (Fig. 2). Nevertheless, the amount of chiloglottone 1 (ng per callus) detected was stage dependent, increasing by developmental stage from traces in young manually opened buds (mo-yb) to amounts in manually opened very mature buds (mo-vmb) that were approx. 25 % of those found in flowers. Interestingly, non-sunlight flowers (nsf) had more chiloglottone 1 than flowers (gdf) that had been depleted of chiloglottone over 3 d following exposure to sunlight for 6 h (Fig. 2).
Mean chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis callus from buds and flowers of different development stages and pre-treatments after 2 h of exposure to sunlight. The insert shows the chemical structure of chiloglottone 1 (2-ethyl-5-propylcyclohexane-1,3-dione). vmb, very mature bud; mo-vyb, manually opened very young bud; mo-yb, manually opened young bud; mo-mb, manually opened mature bud; mo-vmb, manually opened very mature bud; gdf, flowers opened in the growth cabinet lacking UV light (with zero chiloglottone) that were exposed to the sun for 6 h and then returned to the cabinet for depletion over 3 d; nsf, non-sun flowers whereby flowers had opened in the growth cabinet (with zero chiloglottone) and were used 2–3 d after opening. (see Supplementary Data Fig. S1 and Table S1 for the characteristics of the bud stages). Means for each group were estimated based on at least four replicates (range 4–12 replicates per group). Error bars represent the s.e.m. based on pooled estimate of error variance. Labelled columns not connected by the same letter are significantly different at α = 0·05 based on a Tukey–Kramer HSD test. ANOVA: F6,43 = 29·9, P < 0·0001. Chiloglottone 1 amounts were significantly higher in manually opened very mature buds (mo-vmb) compared with the control normal closed very mature bud stage (vmb) for which no chiloglottone 1 was detected. ANOVA: F1,9 = 14·3, P = 0·0043.
Stage dependence and time dependence of chiloglottone 1 production
No chiloglottone 1 was detected in manually opened very young buds (mo-vyb) at any sampling time point (n = 29). For manually opened young buds (mo-yb), chiloglottone was detected in the 180 min (mean ± s.e. = 38·2 ± 13·5 ng per callus; n = 4) and 240 min samples (mean ± s.e. = 18·3 ± 13·5 ng per callus; n = 4), but not at earlier times of sampling. For the remaining treatments (mo-mb, mo-vmb, nsf and gdf), chiloglottone was consistently detected after just 5 min (chiloglottone detection was more variable at 2 min, and showed no correlation with stage or treatment), and showed both stage-dependent and time-dependent increases (Fig. 3).
Time-dependent chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis over 4 h of exposure to sunlight for manually opened buds and flowers and associated best-fit regression lines. mo-mb, manually opened mature bud; mo-vmb, manually opened very mature bud; gdf, depleted flowers that had initially opened in the growth cabinet (with zero chiloglottone) were exposed to the sun for 6 h and then returned to the cabinet lacking UV light for depletion over 3 d; nsf, non-sunlight flowers whereby flowers had opened in the growth cabinet (with zero chiloglottone) and were used 2–3 d after opening (see Supplemenntary Data Fig. S1 and Table S1 for further bud and flower classification details). Regression models [where c1 = chiloglottone 1 amounts (ng per callus), t = time (min)]. mo-mb (c1 = 14·276 + 0·569 × t, R2 = 0·439, F1,30 = 23·50, P < 0·0001); mo-vmb [c1 = 102·169 + 2·585 × t − 0·013 × (t − 66·062)2, R2 = 0·453, F2,35 = 14·468, P < 0·0001]; gdf [c1 = 70·938 + 3·929 × t − 0·011 × (t − 66·062)2, R2 = 0·670, F2,36 = 36·561, P < 0·0001]; nsf [c1 = 129·191 + 5·156 × t − 0·018 × (t − 66·062)2, R2 = 0·696, F2,48 = 55·011, P < 0·0001].
Chiloglottone production over time was higher in the order: mo-mb < mo-vmb < gdf < nsf. Interestingly, regression analysis revealed that the time-dependent increase for the mo-mb was linear, whereas two-degree polynomial regression models were a better fit to the data for the other three cases, reflecting a strong tendency for the mean amount of chiloglottone 1 (ng per callus) to plateau after 180 min (Fig 3). Flowers opened in the growth cabinet (lacking UV-B, so with zero chiloglottone 1) that were then exposed to sunlight for 6 h (producing chiloglottone 1), and then subsequently depleted of chiloglottone back in the UV-B-free growth cabinet, showed a consistent trend of less chiloglottone 1 (ng per callus) compared with flowers that had never produced chiloglottone before (Fig 3). This result is consistent with the trend shown in Fig. 2, where flowers never exposed to sun (nsf) produced more chiloglottone than depleted flowers after 2 h of sunlight exposure.
Chiloglottone 1 production increases with UV-B irradiance
A linear rate of chiloglottone production was detected for mo-mb, whereas two-degree polynomial regression models were a better fit to the data for both the manually opened very mature buds (mo-vmb) and depleted flowers initially sourced from the field (fdf), reflecting a trend for the rates to plateau after the mid irradiance amount (Fig 4). Thus chiloglottone production does increase with UV-B irradiation (up to a point), but with development stage differences in response.
Chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis for increasing UV-B irradiance (µW cm−2 nm−1 at 300 nm) over 2 h of exposure to sunlight for manually opened buds and flowers and associated best-fit regression lines. mo-mb, manually opened mature bud; mo-vmb, manually opened very mature bud; fdf, depleted flower in which flowers had opened in the field and were then maintained in a growth cabinet lacking UV light for depletion to mean chiloglottone amounts of approximately zero (see Supplementary Data Fig. S1 and Table S1 for further bud and flower classification details). Regression models [where c1 = chiloglottone 1 amounts (ng per callus), i = irradiance (µW cm−2 nm−1 at 300 nm)]. mo-mb (c1 =14·516 + 9·687 × i, R2 = 0·436, F1,15 = 11·584, P = 0·004); mo-vmb [c1 = 492·518 + 14·512 × i − 0·0701 × (i − 24·634)2, R2 = 0·404, F2,30 = 10·174, P = 0·0004]; fdf [c1 = 475·119 + 22·707 × i − 0·600 × (i − 24·634)2, R2 = 0·706, F2,16 = 19·265, P < 0·0001].
Relationship between production and starting amounts of chiloglottone 1
The experimental design allowed simultaneous estimation of the amount of chiloglottone 1 (ng per callus) across different stages of depletion, and the amount of chiloglottone at these same stages of depletion following UV-B exposure. If the UV-B-induced chiloglottone production was constant irrespective of the starting amount of chiloglottone 1 (merely reflecting the constant 2 h UV-B exposure time across the experiment), we would predict parallel responses between the two treatments. However, this pattern was not observed. Rather the best-fit linear regression lines for the two treatments diverged significantly across the 7 d experiment (Fig. 5).
Changes in chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis mature flowers on days 1, 3, 5 and 7 of flower incubation in the absence of UV light, followed by either a 2 h UV-B treatment or a control zero UV-B treatment. The best-fit linear regression lines and 95 % confidence intervals (shaded) are also shown for each treatment. Mature C. trapeziformis flowers were sourced from the field and incubated in a growth chamber in the absence of UV light. At the end of each sampling period, ten flowers were sub-sampled and transferred to a UV light box where five samples each were allocated to either the 2 h UV-B (2 h UV) or the zero UV-B control treatment (0 h UV, covered in foil within the light box) at an irradiance of 51 µW cm−2 nm−1 at 300 nm (see also Fig. 4 and Supplementary Data Fig. S4 for irradiance details). Regression models [where c1 = chiloglottone 1 amounts (ng per callus), d = day]. 0 h UV (c1 = 3192·135 − 460·584 × d, R2 = 0·693, F1,17 = 38·413, P < 0·0001); 2 h UV: (c1 = 3422·937 − 246·655 × d, R2 = 0·445, F1,18 = 14·424, P = 0·0013).
Sustained chiloglottone 1 production requires de novo protein synthesis in excised callus
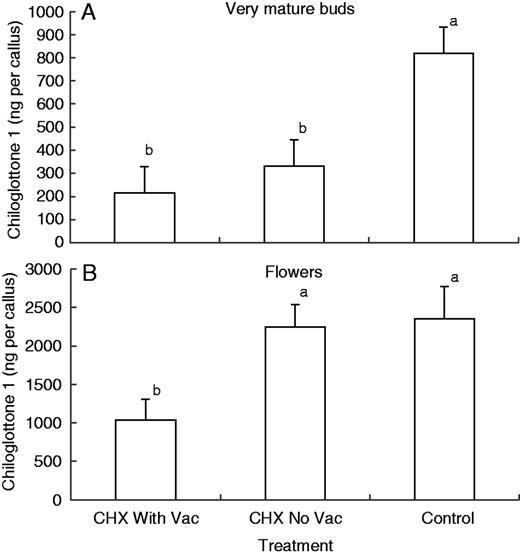
For the very mature buds, the amount of chiloglottone 1 (ng per callus) was significantly lower than the control in both 1 h CHX treatments, with and without vacuum infiltration (Fig 6A). For the depleted flowers, the amount of chiloglottone was only significantly lower in the 1 h CHX treatment with vacuum infiltration.
Mean chiloglottone 1 amounts (ng per callus) within excised Chiloglottis trapeziformis labella from (A) manually opened very mature buds (mo-vmb) and (B) depleted flowers (fdf) for two cychoheximide (CHX) treatments and control followed by 2 h UV-B exposure. Both CHX treatments consisted of 1 h incubation with 100 µM CHX in water containing 0·07 % (v/v) ethanol prior to 2 h UV-B exposure. The CHX With Vac treatment included vacuum infiltration at the start of the incubation period, whereas the CHX No Vac did not. The control consisted of water containing 0·07 % (v/v) ethanol, but no CHX. Note that the irradiance intensity differed approx. 2-fold between the very mature bud treatment (15 W: 23 µW cm−2 nm−1 at 300 nm) and the flower treatments (30 W: 51 µW cm−2 nm−1 at 300 nm). Error bars represent the s.e.m. based on pooled estimate of error variance. Labelled columns not connected by the same letter are significantly different at α = 0·05 based on a Tukey–Kramer HSD test. ANOVA: very mature buds F2,15 = 8·013, P = 0·0043; flowers F2,13 = 5·972, P = 0·0145.
Sustained chiloglottone 1 production requires de novo protein synthesis in cut flowers
Chiloglottone 1 (ng per callus) in the control samples did not change significantly over 72 h (Fig. 7; showing pooled results across two replicates, one replicate each conducted in 2012 and 2013, following confirmation that pooling across the two years was acceptable). In contrast, a strong, significant negative linear relationship between the amount of chiloglottone 1 and hours of CHX incubation was observed.
Changes in chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis mature flowers following incubation with cycloheximide (CHX) for 3, 6, 24 or 72 h, followed by a 2 h UV-B exposure. The best-fit linear regression lines and 95 % confidence intervals (shaded) are shown for both the treatment and control. Mature C. trapeziformis flowers were sourced from the field and incubated in a growth chamber in the absence of UV light for chiloglottone depletion. CHX treatments consisted of incubation with 100 µM CHX in water containing 0·07 % (v/v) ethanol for the specified period, prior to 2 h UV-B exposure at an irradiance of 51 µW cm−2 nm−1 at 300 nm. The controls consisted of water containing 0·07 % (v/v) ethanol, but no CHX. The start point for incubation was timed such that all flowers were sampled 5 d after chiloglottone depletion had commenced. Note that the results shown are based on two experiments run in different years (2012 and 2013), with preliminary statistical analysis confirming pooling was acceptable. Regression models [where c1 = chiloglottone 1 amounts (ng per callus), h = hours]. CHX (c1 = 1440·303 − 13·516 × h, R2 = 0·313, F1,26 = 11·868, P < 0·002); Control (c1 = 2148·356 + 1·923 × h, R2 = 0·445, F1,13 = 0·061, P = 0·809).
Initial chiloglottone 1 production does not require de novo protein synthesis
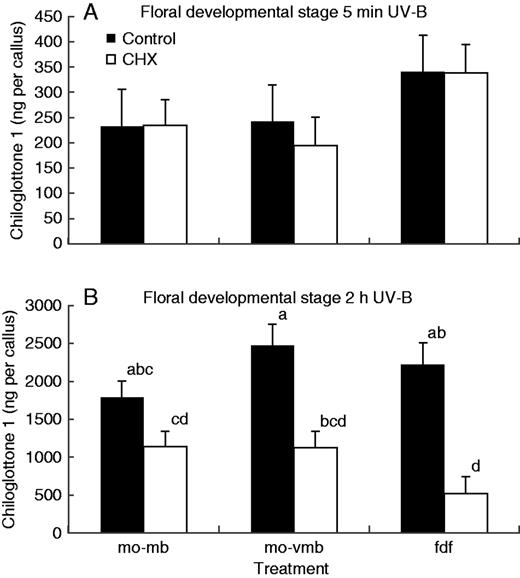
All three floral developmental stages (mo-mb, mo-vmb and fdf) produced measurable chiloglottone 1 amounts within 5 min of UV-B exposure, but no significant differences were detected across developmental stages between the CHX treatment and control (Fig. 8A). In contrast, significant reductions in chiloglottone 1 amounts were detected following the 2 h UV-B exposure (Fig. 8B). Furthermore, the strength of the CHX inhibition was stage specific, being least in manually opened mature buds and greatest in flowers.
Chiloglottone 1 amounts within Chiloglottis trapeziformis callus from manually opened buds and flowers following incubation with cycloheximide (CHX) for 72 h, compared with controls for two UV treatments: (A) 5 min UV-B exposure, (B) 2 h UV-B exposure. mo-mb, manually opened mature bud; mo-vmb, manually opened very mature bud; fdf, depleted flower in which flowers had opened in the field and were then maintained in a growth cabinet lacking UV light for depletion to mean chiloglottone amounts of approximately zero. CHX treatments consisted of incubation with 100 µM CHX in water containing 0·07 % (v/v) ethanol for 72 h, prior to UV-B exposure at an irradiance of 51 µW cm−2 nm−1 at 300 nm for the specified time. The controls consisted of water containing 0·07 % (v/v) ethanol, but no CHX. Means for each group were estimated based on at least three replicates (range 3–6 replicates per group). Error bars represent the s.e.m. based on pooled estimate of error variance. Labelled columns not connected by the same letter are significantly different at α = 0·05 based on a Tukey–Kramer HSD test (graph B only since no significant differences were found in A). ANOVA (A) F5,19 = 0·992, P = 0·449; (B) F5,21 = 9·06, P = 0·0001.
Findings in other Chiloglottis species
Cycloheximide incubation for 72 h did not prevent the initial chiloglottone 1 production upon short (5 min) exposure to UV-B in C. valida or C. seminuda. However, after 2 h of UV-B exposure, chiloglottone amounts were significantly depressed compared with the control (see Supplementary Data Fig. S3). Thus all three Chiloglottis species exhibited similar patterns of response to the CHX and UV-B treatments.
DISCUSSION
Mechanical and developmental control of chiloglottone 1 production
The transmission spectrophotometry revealed that while translucent to visible light, there is no light penetration in the UV-B range through the petal of C. trapeziformis buds (Fig. 1). Furthermore, the sepal and petal tissues physically overlap, at least during the early to mature bud phases, and the callus tissue is further tightly enclosed within the column structure. Thus, UV-B light appears to be completely prevented from reaching the chiloglottone-producing cells in the callus by multiple layers of tissue. Reflectance measurements of Chiloglottis sepals and petals show there is no UV reflectance (Supplementary Data Fig. S5), thus the lack of UV-B light penetration of the floral tissues appears to be entirely due to the strong absorption of UV-B.
The UV-B-absorbing properties of plant leaves are well established (Rozema et al., 2002; Noda et al., 2013; Klancnik et al., 2014; Robson et al., 2014). Similarly, flower parts, such as sepals and petals are well known for both their UV reflection and absorption properties (Silberglied, 1979). Often UV-absorbing pigments, such a flavonoids, are arranged in flowers so as to provide strong floral markings, or nectar guides, that are visible to insects that can see in the UV range, but invisible to humans (Gronquist, 2001; Schlangen et al., 2009). In the case of Chiloglottis, the petals appear to be uniformly and strongly absorbing in the UV-B range.
Given the UV-B light-blocking capability of the outer floral tissues of the bud, it is possible that mechanical blocking alone could explain the lack of chiloglottone 1 detected in buds (Falara et al., 2013; this study). Indeed, when buds where manually opened to allow exposure of the labellum to sunlight, chiloglottone was detected in the callus tissue in all but the very young bud, and increased with developmental stage (Fig. 2). In subsequent experiments involving manually opened buds at the mature (mo-mb) and very mature (mo-vmb) phases, chiloglottone production was consistently detected following UV-B exposure (Figs 3, 4 and 8). Furthermore, while these two mature bud phases both exhibited increasing chiloglottone with increasing time of UV-B exposure and increasing UV-B light intensity, the maximum chiloglottone 1 amounts were capped, consistent with a stage-dependent maximum amount of synthesis or storage (Figs 3 and 4). Thus in nature, it would appear that the mere opening of the mature bud into a flower exposes the chiloglottone-producing tissues to the essential UV-B radiation, which quickly causes commencement of chiloglottone production, with chiloglottone increasing over time as the flower matures.
UV-B contribution to chiloglottone 1 production and the role of de novo protein synthesis
A time-dependent increase in chiloglottone production occurs over 2 h in sunlight, using depleted flowers (Falara et al., 2013). Here flowers and buds were exposured to sunlight over a longer time course of 4 h, and to different intensities of UV-B. The amount of chiloglottone 1 (ng per callus) increased with both increasing exposure to sunlight (Fig. 3) and increasing UV-B irradiance (Fig. 4), with the maximum reached consistently reflecting the same pattern of stage dependence across the two experiments (mo-mb > mo-vmb > flowers). However, while statistical analysis revealed the response of the manually opened mature buds (mo-mb) to be linear for both time in the sun and UV-B irradiance, the responses of manually opened very mature buds (mo-vmb) and flowers reached a plateau in both experiments (Figs 3 and 4). This suggests that a common negative feedback control loop on synthesis or storage of the free chiloglottone 1 is operating in very mature buds and flowers, irrespective of whether chiloglottone increases in response to time of exposure or irradiance. The present lack of evidence for a plateau in response in the mature buds could indicate that the feedback mechanism has not yet fully developed, or simply that the amount of chiloglottone 1 had not yet reached a critical threshold.
The present study confirmed that the previously known rapid production of chiloglottone within minutes of UV-B exposure in depleted flowers (Falara et al., 2013) also occurs in manually opened mature buds (Figs 3, 6 and 8). Since in this short time (5 min) it is unlikely that induction of gene transcription and translation had an effect on the availability of biosynthetic enzymes, this suggests that some of the enzymes and substrates required are immediately available to initiate chiloglottone production when UV-B light is present, and therefore that one of the roles of UV-B might be an involvement in the reaction itself. However, the lag time to reach maximum chiloglottone amounts suggests that protein synthesis might also be required.
To assess this possibility, we employed the de novo protein synthesis inhibitor, CHX (Siegel and Sisler, 1963; Ellis and MacDonald, 1970; Kurepa et al., 2010; Guirimand et al., 2012), noting that the mode of action is still not fully understood (Schneider-Poetsch et al., 2010), and that caution is required because secondary effects beyond protein inhibition are known (McMahon, 1975; Oksvold et al., 2012). Nevertheless, CHX treatments can induce rapid and reversible inhibition of de novo protein synthesis with incubation times as short as 1 h when the inhibitor can quickly reach the target tissues, such as cell cultures (e.g. Abebie et al., 2008; Chung et al., 2008; Guirimand et al., 2012). Longer incubation times of several hours may be necessary when working with whole-plant systems where it can take time for the CHX to be delivered to the tissues of interest (Sultan and Farooq, 1997; Truitt et al., 2004). However, it is essential to develop experiments that optimize the uptake of the CHX so as to detect direct effects (if any) as quickly as possible.
In the first two CHX experiments, we worked with excised labellum callus incubated in solutions containing the inhibitor. For callus tissue sourced from very mature buds, significant reductions in chiloglottone 1 were detected in just 1 h of CHX incubation, irrespective of whether or not the callus tissue was treated by vacuum infiltration (Fig. 6A). In contrast, for callus tissue from depleted flowers, only with vacuum infiltration was a significant reduction detected (Fig. 6B). This observed difference between floral developmental stages perhaps reflects the ease of CHX penetration into the tissue of the younger tissue. Nevertheless, in both experiments, the rapid inhibition of chiloglottone production within 1 h of CHX inhibition strongly indicates that de novo protein synthesis is required to achieve maximum chiloglottone production over 2 h of UV-B exposure.
In a subsequent experiment, in which the CHX was delivered to intact flowers via the stalk, a significant time-dependent decrease in chiloglottone production was detected for incubation times varying from 3 to 72 h (Fig. 7). These results are consistent with rapid and increasing de novo protein synthesis inhibition.
In a final experiment to tease out whether or not de novo protein synthesis is required to initiate chiloglottone production following UV-B induction, the amount of chiloglottone 1 (ng per callus) after 72 h of CHX incubation was measured after 5 min vs. 2 h of UV-B exposure. Despite the long incubation time for CHX, no significant difference in chiloglottone 1 was detected after 5 min of UV-B exposure, whereas strong reductions in the amount of chiloglottone were detected after 2 h of UV-B exposure (Fig. 8). Thus the findings further support the hypothesis that some enzymes and substrates are available to support initial chiloglottone production. However, de novo protein synthesis does appear to be critical to sustain chiloglottone production beyond initiation of production.
Metabolic control and feedback loops in chiloglottone 1 production
Our findings reveal critical new insights into the link between UV-B and chiloglottone production. Within the buds, chiloglottone production appears to be controlled by the combination of mechanical and developmental regulation. In the first instance, the physical opening of the bud enables the UV-B in natural sunlight to reach the chiloglottone-producing tissue, rapidly causing chiloglottone production, with production probably increasing as the flower matures.
Several lines of experimental evidence suggest negative feedback control of the internal amount of chiloglottone 1 in the flower. These include: (1) the trend for depleted flowers to exhibit lower maximum amounts of chiloglottone than flowers not previously exposed to sunlight (Figs 2 and 3); (2) the common finding that manually opened very mature buds (mo-vmb) and depleted flowers (fdf) both showed a plateau in production after 3 h of sunlight, and at higher UV-B irradiances (Figs 3 and 4); and (3) the disproportionate increase in chiloglottone production after 2 h UV-B exposure with decreasing chiloglottone (depletion) (Fig. 5).
Of additional interest is that the depletion rate appears to be linear (with neither log or polynomial models offering better fits over the linear regression model) at an average of 19·2 ng per callus h−1 (based on the regression equation for 0 h UV in Fig. 5 with a rate of −460·584 ng per callus d−1). In contrast, the maximum chiloglottone 1 production rate over 2 h of UV-B exposure on day 7 was estimated at 864·2 ng per callus h−1 (based on the difference between regression lines in Fig. 5). Also of interest are the high internal chiloglottone 1 amounts (ng per callus) apparent in this and subsequent experiments conducted during the mid-flowering period in 2013. At these unusually high chiloglottone amounts, up to 7 d was required to achieve full depletion, whereas in earlier experiments in the study when chiloglottone 1 was less (e.g. Figs 2–4), depletion to zero was achieved in 3–5 d. The reason for the observed variability across the flowering period is unknown.
One difficulty for inferring that there is feedback control of chiloglottone production is the inability to measure the emission rate of chiloglottone in the headspace. For example, the inference from the experiments shown in Fig. 5 is that chiloglottone production increases with decreasing starting amounts of chiloglottone 1. However, we cannot fully rule out the alternative possibility that production is constant, with any chiloglottone excess above some internal storage capacity being either emitted or stored in some other form. Under this scenario, the best estimate of the maximum chiloglottone production rate is provided when chiloglottone 1 is depleted (864 ng per callus h−1; see above). An alternative estimate of a minimum steady-state emission might be provided by the chiloglottone depletion rate (assuming depletion is entirely due to emission) of 19 ng per callus h−1 (see above), which is 45 times less than the estimated maximum production rate. Given our inability to detect chiloglottone in the headspace, a minimum steady-state rate of chiloglottone production estimated from the depletion rate is the most realistic estimate.
Conclusions
Our study has shown for the first time that the floral tissues of Chiloglottis orchids strongly block UV-B wavelengths of light, but that manually opened buds can produce chiloglottone 1. Furthermore, while some chiloglottone biosynthesis requires only UV-B light but no protein synthesis, sustained chiloglottone biosynthesis over a 2–4 h period requires both UV-B and protein biosynthesis. We have also shown that the amount of chiloglottone 1 found in flowers reflects the interplay between developmental stage, time and intensity of UV-B exposure, de novo protein synthesis, and feedback loops that cap the internal amount. Our findings confirm a critical role for UV-B in both the initiation and maintenance of chiloglottone production and indicate that UV-B contributes directly to the chiloglottone biosynthesis itself.
ACKNOWLEDGEMENTS
We thank the Australian National Botanic Gardens, Canberra for permission and permits to take plant material from the gardens, and Dr Rudy Dolferous (CSIRO Plant Industry) for providing cycloheximide. We also thank Dr Col Bower for sharing his knowledge of wild orchid locations, and Dr Ryan Phillips (EEG, ANU) and Suban Sanoubane for assistance with the field sampling of C. seminuda flowers. The flower samples were collected under a permit provided by the National Parks and Wildlife Service of New South Wales to RP. Dr Marilyn Ball and the editor, Dr David Lawlor, provided insightful comments that improved the manuscript. This work was supported by Australian Research Council projects DP0451374 to R.P., and DP1094453 to R.P., R.A.B. and E.P.
LITERATURE CITED




![Time-dependent chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis over 4 h of exposure to sunlight for manually opened buds and flowers and associated best-fit regression lines. mo-mb, manually opened mature bud; mo-vmb, manually opened very mature bud; gdf, depleted flowers that had initially opened in the growth cabinet (with zero chiloglottone) were exposed to the sun for 6 h and then returned to the cabinet lacking UV light for depletion over 3 d; nsf, non-sunlight flowers whereby flowers had opened in the growth cabinet (with zero chiloglottone) and were used 2–3 d after opening (see Supplemenntary Data Fig. S1 and Table S1 for further bud and flower classification details). Regression models [where c1 = chiloglottone 1 amounts (ng per callus), t = time (min)]. mo-mb (c1 = 14·276 + 0·569 × t, R2 = 0·439, F1,30 = 23·50, P < 0·0001); mo-vmb [c1 = 102·169 + 2·585 × t − 0·013 × (t − 66·062)2, R2 = 0·453, F2,35 = 14·468, P < 0·0001]; gdf [c1 = 70·938 + 3·929 × t − 0·011 × (t − 66·062)2, R2 = 0·670, F2,36 = 36·561, P < 0·0001]; nsf [c1 = 129·191 + 5·156 × t − 0·018 × (t − 66·062)2, R2 = 0·696, F2,48 = 55·011, P < 0·0001].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/aob/115/4/10.1093_aob_mcu262/5/m_mcu262f3p.jpeg?Expires=1716411156&Signature=PAikcdC8gZ2KMtSGAg3ONDzZvX5OLNh2-FpIthPmMqDwUDXafRg9tXzq25eOYhg5maJbQRstg79HeXzIGVoDlNu6RgwzgbfWRzTnkru7MvbArr~CWoj7e6Dcp6l86GY7g9wudvHH7nFv6S9nh50PnCK-a8hwYpModNKx-sPypyKGSfSgTZRMjqk8lS9XFpzPBg75VspeRaj9Zd7x-0OpHaDT2wpe555Tf5-hb-hlQwmhYIPGPLmrDXoctUf1XpmnSWhBaHdYHyZW5JCshM-0KSBWymUDJD3kS~Qt3R4UYAfAFbSGn9jdfAGETE56Sr-ez5v-grAKG9MCMBuCPhdm~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis for increasing UV-B irradiance (µW cm−2 nm−1 at 300 nm) over 2 h of exposure to sunlight for manually opened buds and flowers and associated best-fit regression lines. mo-mb, manually opened mature bud; mo-vmb, manually opened very mature bud; fdf, depleted flower in which flowers had opened in the field and were then maintained in a growth cabinet lacking UV light for depletion to mean chiloglottone amounts of approximately zero (see Supplementary Data Fig. S1 and Table S1 for further bud and flower classification details). Regression models [where c1 = chiloglottone 1 amounts (ng per callus), i = irradiance (µW cm−2 nm−1 at 300 nm)]. mo-mb (c1 =14·516 + 9·687 × i, R2 = 0·436, F1,15 = 11·584, P = 0·004); mo-vmb [c1 = 492·518 + 14·512 × i − 0·0701 × (i − 24·634)2, R2 = 0·404, F2,30 = 10·174, P = 0·0004]; fdf [c1 = 475·119 + 22·707 × i − 0·600 × (i − 24·634)2, R2 = 0·706, F2,16 = 19·265, P < 0·0001].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/aob/115/4/10.1093_aob_mcu262/5/m_mcu262f4p.jpeg?Expires=1716411156&Signature=U-kC8uWT0udhUcoISFXg3SuaWdwBOL2peEu9HuxAT7o6mm9VvElVrqgYRx-wjb4uqJPGYMi-Sc2UbTHnrwW1qDBvmQWVGN45vV-8enmIgTVHif23AxM~XEmrcyC9aa~uwznsxyquC~sCIbht6FFGoqw4ReqlN0wlbTROv8JnptVEGHuDt3ErVWQfqZAqjlp2P7SewX6xyS~TTcANrLQChAm6fE8ZBMGmjefFP5LlHeSgJ-zIiThuiIdIuo88D9OLhnmnt7VcRo10zNWHm5OT3DHl8xskz6XrTrLvM9xtLtg~PZGsBrsQliZWASENo7l85UTS8qHnQ5Yc3n6YbIBU-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Changes in chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis mature flowers on days 1, 3, 5 and 7 of flower incubation in the absence of UV light, followed by either a 2 h UV-B treatment or a control zero UV-B treatment. The best-fit linear regression lines and 95 % confidence intervals (shaded) are also shown for each treatment. Mature C. trapeziformis flowers were sourced from the field and incubated in a growth chamber in the absence of UV light. At the end of each sampling period, ten flowers were sub-sampled and transferred to a UV light box where five samples each were allocated to either the 2 h UV-B (2 h UV) or the zero UV-B control treatment (0 h UV, covered in foil within the light box) at an irradiance of 51 µW cm−2 nm−1 at 300 nm (see also Fig. 4 and Supplementary Data Fig. S4 for irradiance details). Regression models [where c1 = chiloglottone 1 amounts (ng per callus), d = day]. 0 h UV (c1 = 3192·135 − 460·584 × d, R2 = 0·693, F1,17 = 38·413, P < 0·0001); 2 h UV: (c1 = 3422·937 − 246·655 × d, R2 = 0·445, F1,18 = 14·424, P = 0·0013).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/aob/115/4/10.1093_aob_mcu262/5/m_mcu262f5p.jpeg?Expires=1716411156&Signature=HZvZLHfZuq4jqquCHZJmq21HpzmynOCeL5LdWL8ZBddiU6yxs2x8y~1Fr-E-tEsN9uEZ70yYEWBL3BliiLFTx9TDckyZ-OH9rKqsXsRcHZXQYObJWueqGVYQwmO4cz5mmgnwdshtxKH46NxgQskavFYZ09iDm4t~va-O6jKmEA8oTYQGWR1fA1PAH~JQNrSlmIhVOPkS~mWqpcDYsbv-MQNr00CcqPao9OZFGnTm7IXpa7eo8pl3x4rR0JaAd6xShCI4LO~GWmirL9XRLue5ayYvjEN7rn-~1CDZr35cEZKj3KNJc~nEWvm3ktRWRkvre2IhWPouKVC6334Ee25Gbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Changes in chiloglottone 1 amounts (ng per callus) within Chiloglottis trapeziformis mature flowers following incubation with cycloheximide (CHX) for 3, 6, 24 or 72 h, followed by a 2 h UV-B exposure. The best-fit linear regression lines and 95 % confidence intervals (shaded) are shown for both the treatment and control. Mature C. trapeziformis flowers were sourced from the field and incubated in a growth chamber in the absence of UV light for chiloglottone depletion. CHX treatments consisted of incubation with 100 µM CHX in water containing 0·07 % (v/v) ethanol for the specified period, prior to 2 h UV-B exposure at an irradiance of 51 µW cm−2 nm−1 at 300 nm. The controls consisted of water containing 0·07 % (v/v) ethanol, but no CHX. The start point for incubation was timed such that all flowers were sampled 5 d after chiloglottone depletion had commenced. Note that the results shown are based on two experiments run in different years (2012 and 2013), with preliminary statistical analysis confirming pooling was acceptable. Regression models [where c1 = chiloglottone 1 amounts (ng per callus), h = hours]. CHX (c1 = 1440·303 − 13·516 × h, R2 = 0·313, F1,26 = 11·868, P < 0·002); Control (c1 = 2148·356 + 1·923 × h, R2 = 0·445, F1,13 = 0·061, P = 0·809).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/aob/115/4/10.1093_aob_mcu262/5/m_mcu262f7p.jpeg?Expires=1716411156&Signature=eOvvTj7QHtYp7EkOklFbE46Kkd16qZDiFQbACG2iGGRMgPAewj9TOwQQYjKa9fZa5r86SpQWhg7tTgSSNCJL2PZT6O8ZevkuQHUmhDWgSXJptba3zKIA65mny1OgGHihWGMx7aYMFnlOTCSAImyeacVZecT-m0Lx5WP5cJIj5NADxDLmo3I-VosfVqSW6nm8csUbnI75WU1OfchMGQmJBRrAHumSmDFOdR6ARhxrwb9mwEgPVjUUAfjcCsLxZ9z6GE0YIHpAJfcY1XY802WJMz1r5dqY0P8ZG-ADfMA~QAevK3gKLJUZqOJMwpzvRrstohCa5zG2cdKsnu-NGCih3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)