-
PDF
- Split View
-
Views
-
Cite
Cite
Nicolay Leme da Cunha, Erich Fischer, Aline P. Lorenz-Lemke, Spencer C. H. Barrett, Floral variation and environmental heterogeneity in a tristylous clonal aquatic of the Pantanal wetlands of Brazil, Annals of Botany, Volume 114, Issue 8, December 2014, Pages 1637–1649, https://doi.org/10.1093/aob/mcu181
Close - Share Icon Share
Abstract
The balance between stochastic forces and frequency-dependent mating largely governs style morph frequencies in heterostylous populations. In clonal species, deviations from equal morph ratios often result from founder events and unfavourable conditions for sexual reproduction. The aim of this study was to investigate whether different flooding regimes, because of their influence on sexual vs. clonal reproduction, are associated with regional variation in morph frequencies and floral trait differentiation in populations of the clonal, tristylous, aquatic Eichhornia azurea (Pontederiaceae) in the Pantanal wetlands of Brazil.
Style morph frequencies were sampled from 73 populations distributed across four flooding regimes differing in depth and duration. Measurements of flower size, sex-organ dimension, pollen size and pollen production were made in selected populations, and pollinator assemblages and their functional traits were recorded.
Most populations of E. azurea were tristylous (78 %), but the majority exhibited uneven morph ratios. The frequency of the mid-styled morph was significantly lower than that of the long- and short-styled morphs. Morph evenness was positively associated with population size but not with flooding regime. There were significant phenotypic differences among flooding regimes for all floral traits, including populations with reduced flower size, sex-organ length and smaller pollen. Pollinator assemblages varied with flood duration.
The similar morph structure and evenness of populations, regardless of flooding regime, suggest that sexual reproduction and clonal dispersal are sufficiently common to prevent the signature of founder events from dominating in a region. However, the pervasive occurrence of biased morph ratios in most populations suggests that many are in a non-equilibrium state. The reduced frequency of the mid-styled morph in trimorphic and dimorphic populations may be associated with the weak self-incompatibility of this morph resulting in selfing and inbreeding depression. Clonality in E. azurea and the weak self-incompatibility of the mid-styled morph may make it more vulnerable to geitonogamous selfing.
INTRODUCTION
The relative frequency of sexual and asexual reproduction in many clonal plant populations is largely governed by environmental factors. Conditions permitting sexual reproduction are usually more restricted than those favouring vegetative growth and clonal propagation (Harper, 1977; Jackson et al., 1985). Stressful abiotic conditions affecting flowering and fruiting, low pollinator service resulting in pollen limitation, and the absence of suitable conditions for seed germination and seedling establishment can each limit the degree of sexual reproduction in clonal populations, particularly those that occupy aquatic environments (Sculthorpe, 1967; Hutchinson, 1975; Barrett, 1980; Philbrick and Les, 1996). In contrast, as long as environmental conditions allow the persistence of populations, vegetative growth usually enables some degree of clonal propagation to occur. Although genetic factors, such as sterility and the absence of mating partners (reviewed in Barrett et al., 1993; Eckert, 2002), can influence the relative importance of sexual and asexual reproduction in clonal species, environmental heterogeneity probably plays a more important role in governing the balance between these contrasting reproductive strategies.
Determining the relative frequency of sexual vs. asexual reproduction in plant populations is a challenging problem. This is because direct estimates of reproductive success require long-term demographic studies of the fates of clonal propagules and seedlings, and this is rarely undertaken. More commonly, inferences on the relative importance of these reproductive modes are made using genetic markers (Arnaud-Haond et al., 2005; Harada et al., 1997). Measurements of clonal (genotypic) diversity and clone size are commonly used to assess the relative amounts of sexual vs. clonal propagation (Ellstrand and Roose, 1987; Silvertown, 2008). Plant species that are sexually polymorphic can also be used to infer the relative importance of clonal and sexual reproduction, based on visual inspection of the frequency of mating types in populations. For example, in dioecious species, regular sexual reproduction should result in equal frequencies of the sex phenotypes in equilibrium populations. Similarly, sustained disassortative mating between style morphs in species with stylar polymorphisms should result in equal floral morph ratios. However, prolific clonal propagation can frequently lead to biased sex ratios in dioecious populations (Field et al., 2013), and to anisoplethic (uneven) floral morph ratios in heterostylous (Wang et al., 2005) and enantiostylous (Jesson and Barrett, 2002) populations. If ecological conditions restrict sexual recruitment, founder events can play an important role in affecting sex or morph ratios in species with sexual polymorphisms.
Tristyly is a form of heterostyly in which populations are typically composed of three style morphs that differ reciprocally in stigma height and anther level (Darwin, 1877). In large equilibrium populations, in which there are no fitness differences between the morphs, a 1 : 1 : 1 (isoplethic) style morph ratio should occur as a result of negative frequency-dependent selection (Barrett, 1993). However, tristylous species often propagate clonally, and limited sexual recruitment governed by ecological factors can result in slow progress towards equal morph ratios. As a consequence, deviations from isoplethy are commonly reported in clonal populations of tristylous species (e.g. Decodon, Eckert and Barrett, 1992; Eichhornia, Barrett, 1977; Pontederia, Morgan and Barrett, 1988; Oxalis, Ornduff, 1972). In some cases, the dispersal of clonal propagules followed by extensive vegetative growth can give rise to populations that contain only one (monomorphic) or two (dimorphic) style morphs (Ornduff, 1972; Barrett and Forno, 1982; Eckert and Barrett, 1995; Castro et al., 2013). Whether non-trimorphic populations persist, or become trimorphic, depends on a variety of factors, including the compatibility status of floral morphs, whether gene flow restores missing morphs and the extent to which local environmental conditions allow opportunities for sexual reproduction.
Here, we investigate the floral biology of the bee-pollinated, tristylous, clonal aquatic Eichhornia azurea in the Pantanal wetlands of Brazil. Earlier studies of E. azurea established that clonal propagation is a pervasive feature of populations and that style morph ratios vary considerably, including the occurrence of monomorphic populations and those in which selfing variants (semi-homostyly) occur (Barrett, 1978; Alvos dos Santos, 2002). The flowers of semi-homostylous variants usually differ from those of tristylous morphs in being smaller and in possessing one set of stamens adjacent to the stigma facilitating autonomous self-pollination (Ornduff, 1972; Barrett, 1988). Eichhornia azurea is particularly common in the Pantanal wetlands, occupying a diversity of aquatic habitats that differ in flooding regimes and opportunities for sexual reproduction (Cunha and Fischer, 2009). In many aquatic species, including species of Eichhornia, the occurrence of sexual reproduction is dependent on the availability of safe sites for seed germination and seedling establishment, and these often correspond to periods of low water level (Sculthorpe, 1967; Barrett, 1980). The striking environmental heterogeneity that characterizes the Pantanal wetlands therefore provides an outstanding opportunity to investigate the influence of ecological conditions on the maintenance of a genetic polymorphism, and whether different flooding regimes may be associated with regional variation in morph frequencies and floral traits. In addition, if flooding regimes also differentially influence the distribution of bee species in the Pantanal, it is possible that populations of E. azurea in different regions of the Pantanal may be visited by contrasting groups of pollinators that vary in their effectiveness in promoting disassortative mating and thus potentially influencing the evolution of floral traits. We therefore also investigated whether there was evidence of regional variation in pollinator assemblages visiting populations and whether this might be associated with the phenotypic differentiation of floral traits.
Our study of E. azurea addressed the following specific questions. (1) What are the patterns of variation in style morph frequencies in populations, and do they differ among flooding regimes? We predicted more biased morph ratios in regions with longer flood periods owing to the possibility of more limited opportunities for sexual recruitment. (2) What is the relationship between population size and deviations from isoplethy (morph evenness)? We predicted that stochastic forces such as founder effects would be more evident in small populations and therefore they would be more likely to exhibit uneven morph ratios compared with populations of larger size. (3) Is there evidence for regional phenotypic differentiation of floral traits within the Pantanal, specifically variation in flower size, sex-organ dimensions, pollen size and pollen production? We investigated the extent to which flooding regimes and pollinator assemblages may be associated with floral differentiation. Specifically, we were interested in determining whether there was evidence for the breakdown of tristylous floral traits, including reduced flower size, greater variation in sex-organ placement, reduction in pollen size trimorphism and the possible occurrence of semi-homostyly. These floral modifications have been reported in other tristylous Eichhornia species (reviewed in Barrett, 1988), under conditions in which effective cross-pollination and disassortative mating is restricted because of inferior pollinator service.
MATERIALS AND METHODS
Study region
The Pantanal is located in the centre of South America (15–20 °S) and is one of the largest wetlands on Earth, covering approx. 135 000 km2 of floodplains (Silva and Abdon, 1998; Assine, 2003), with a total area of 160 000 km2 (Junk and Nunes da Cunha, 2005) and an altitude of 80–190 m. The Pantanal originated during the transition between the Pleistocene and Holocene, and has experienced several climatic changes during its history: 40 000–8000 years ago, cold and dry; 8000–3500 years ago, hot and wet; 3500–1500 years ago, hot and dry; and 1500 years ago to the present day, hot and wet (Iriondo and Garcia, 1993; Stevaux, 2000). Alternations between wet and dry periods have led to different patterns of sediment deposition in the Paraguay River and its tributaries, resulting in a mosaic of distinct geomorphological formations now covered by various vegetation types (Short and Blair, 1986; Rueda et al., 1998).
The most significant factor currently influencing the Pantanal is the unimodal, annual flood pulse, which shows considerable spatial and temporal variation (Hamilton et al., 1996; Silva and Abdon, 1998; Hamilton, 2002; Gonçalves et al., 2011). During rainy periods, precipitation in the surrounding plateau regions drains into the Pantanal causing extensive flooding. Water flows slowly from east to west in permanent and temporary streams and then southwards upon entering the Paraguay River. Seasonal fluctuations in water level generally range from 2 to 5 m in the Paraguay River, but typically have lower values across the Pantanal. The climate of the Pantanal is tropical, with a well-defined dry (May–October) and wet season (November–April), and annual precipitation ranges from 1000 to 1500 mm, mainly between November and March.
The Pantanal is composed of several sub-regions with distinct geomorphological, hydrological and ecological characteristics. The number of sub-regions and criteria used to distinguish them differ (e.g. EDIBAP, 1979; Adámoli, 1986; RADAMBRASIL, 1982), but here we follow the classification of Hamilton et al. (1996) based on hydrological and geomorphological features. In our study, we classified all sampled populations of E. azurea according to four major flooding regimes typical of the habitats they occupied, using available information in the literature (Hamilton et al., 1996, Gonçalves et al., 2011) and our own observations. The four regimes differ according to the height and duration of the annual flood pulses; they are hereafter referred to as low and short (LS), medium and medium (MM), medium and long (ML), and high and long (HL). Each flood regime tends to characterize a particular geographical region of the Pantanal (Table 1).
Flood regimes among four regions of the Pantanal wetlands of Brazil and the flowering season of Eichhornia azurea in each region
| Flood class (height–duration) . | Regions . | Water depth (m) . | Duration of peak flood (months) . | Water origin . | Habitats . | Connection among water bodies . | Eichhornia azurea flowering period . |
|---|---|---|---|---|---|---|---|
| Low–Short (LS) | Leque do Taquari, Nabileque, Aquidauana-east | 0·1–1 | 2–4 | Mainly precipitation and less river flood | Swamps and temporary lakes | Temporary channels | February–June |
| Medium–Medium (MM) | Nhecolândia | 0·5–1·5 | 4–5 | Precipitation and river flood | Swamps, temporary and permanent lakes | Temporary channels | October–June |
| Medium–Long (ML) | Miranda, Aquidauana-west | 1–3 | 4–6 | Mainly river flood and less precipitation* | Swamps, temporary and permanent lakes, rivers | Temporary channels | Year round |
| High–Long (HL) | Paraguay | 1–6 | 6 | Mainly flood | Permanent lakes and river | Permanent channels | Year round |
| Flood class (height–duration) . | Regions . | Water depth (m) . | Duration of peak flood (months) . | Water origin . | Habitats . | Connection among water bodies . | Eichhornia azurea flowering period . |
|---|---|---|---|---|---|---|---|
| Low–Short (LS) | Leque do Taquari, Nabileque, Aquidauana-east | 0·1–1 | 2–4 | Mainly precipitation and less river flood | Swamps and temporary lakes | Temporary channels | February–June |
| Medium–Medium (MM) | Nhecolândia | 0·5–1·5 | 4–5 | Precipitation and river flood | Swamps, temporary and permanent lakes | Temporary channels | October–June |
| Medium–Long (ML) | Miranda, Aquidauana-west | 1–3 | 4–6 | Mainly river flood and less precipitation* | Swamps, temporary and permanent lakes, rivers | Temporary channels | Year round |
| High–Long (HL) | Paraguay | 1–6 | 6 | Mainly flood | Permanent lakes and river | Permanent channels | Year round |
*Winter precipitation promotes irregular floods.
Flood regimes among four regions of the Pantanal wetlands of Brazil and the flowering season of Eichhornia azurea in each region
| Flood class (height–duration) . | Regions . | Water depth (m) . | Duration of peak flood (months) . | Water origin . | Habitats . | Connection among water bodies . | Eichhornia azurea flowering period . |
|---|---|---|---|---|---|---|---|
| Low–Short (LS) | Leque do Taquari, Nabileque, Aquidauana-east | 0·1–1 | 2–4 | Mainly precipitation and less river flood | Swamps and temporary lakes | Temporary channels | February–June |
| Medium–Medium (MM) | Nhecolândia | 0·5–1·5 | 4–5 | Precipitation and river flood | Swamps, temporary and permanent lakes | Temporary channels | October–June |
| Medium–Long (ML) | Miranda, Aquidauana-west | 1–3 | 4–6 | Mainly river flood and less precipitation* | Swamps, temporary and permanent lakes, rivers | Temporary channels | Year round |
| High–Long (HL) | Paraguay | 1–6 | 6 | Mainly flood | Permanent lakes and river | Permanent channels | Year round |
| Flood class (height–duration) . | Regions . | Water depth (m) . | Duration of peak flood (months) . | Water origin . | Habitats . | Connection among water bodies . | Eichhornia azurea flowering period . |
|---|---|---|---|---|---|---|---|
| Low–Short (LS) | Leque do Taquari, Nabileque, Aquidauana-east | 0·1–1 | 2–4 | Mainly precipitation and less river flood | Swamps and temporary lakes | Temporary channels | February–June |
| Medium–Medium (MM) | Nhecolândia | 0·5–1·5 | 4–5 | Precipitation and river flood | Swamps, temporary and permanent lakes | Temporary channels | October–June |
| Medium–Long (ML) | Miranda, Aquidauana-west | 1–3 | 4–6 | Mainly river flood and less precipitation* | Swamps, temporary and permanent lakes, rivers | Temporary channels | Year round |
| High–Long (HL) | Paraguay | 1–6 | 6 | Mainly flood | Permanent lakes and river | Permanent channels | Year round |
*Winter precipitation promotes irregular floods.
Study species
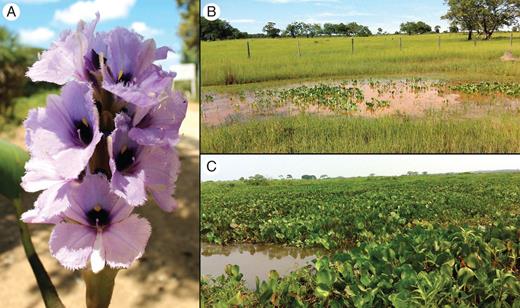
Eichhornia azurea (Pontederiaceae) is a rhizomatous, clonal perennial that is commonly attached to the sediment (‘floating leaved aquatic’ sensuSculthorpe, 1967), although dispersal by free-floating clonal stem fragments is common. The species is widely distributed throughout the lowland Neotropics from Argentina to Central America and the Caribbean, occupying diverse aquatic habitats (Barrett, 1978; Bianchi et al., 2000; Alves dos Santos, 2002; Cunha and Fischer, 2009). The showy flowers of E. azurea are composed of six fimbriate petals and a well-developed floral tube, and are produced in inflorescences of between 20 and 100 flowers. Flowers range in colour from pale blue to purple, with a prominent yellow nectar guide on an azure blue background above the throat of the floral tube (Fig. 1). Flowers open for 6–12 h depending on temperature, and inflorescences are in bloom for between 2 and 5 d. In the Pantanal, E. azurea can flower year round, depending on the region and water availability (Table 1). Flowers are visited by bees, flies and occasional butterflies; the long-tongued anthophorid bee Ancyloscelis gigas is of most importance in promoting cross-pollination (Barrett, 1978; Alves dos Santos and Wittmann, 2000; Alves dos Santos, 2002). After flowering, the inflorescence axis bends downward below the surface of the water (geniculation), and fruit and seed development occurs under water until dispersal approx. 20–30 d after pollination. The fruits are capsules containing up to 100 seeds.
Eichhornia azurea in the Pantanal wetlands of Brazil. (A) Inflorescence of the long-styled morph; (B) population in the flooding regime low depth and short duration (LS; see Table 1); (C) population in the flooding regime high depth and long duration (HL).
Eichhornia azurea possesses the floral polymorphism tristyly (reviewed in Barrett, 1993), with long- mid- and short-styled floral morphs (hereafter L-, M- and S-morphs). Tristyly in E. azurea is associated with a trimorphic self-incompatibility system and pollen size trimorphism (Barrett, 1978; Alves dos Santos and Wittmann, 2000; Bianchi et al., 2000), and, in common with other tristylous members of the Pontederiaceae with trimorphic incompatibility (see Barrett and Anderson, 1985; Puentes et al., 2013), the M-morph of E. azurea exhibits a weaker incompatibility system than the L- and S-morphs, allowing considerable numbers of seeds to be produced from hand self-pollination (Alves dos Santos and Wittmann, 2000; Bianchi et al., 2000; Cunha and Fischer, 2009). Semi-homostylous, self-compatible forms of E. azurea are reported from Costa Rica and southern Brazil as a result of the evolutionary breakdown of tristyly (Barrett, 1978; Alves dos Santos, 2002).
Sampling of floral variation
To investigate floral variation in E. azurea, we sampled populations in different regions of the Pantanal from 2004 to 2012, and also added four populations from the literature. We sampled populations from the four types of flooding regime described in Table 1 in order to maximize the range of environmental conditions in which populations occurred. The habitats in which populations were found included both permanent and temporary lakes, and channels, ditches, flooded pastures and swamps. For the purpose of this study, we define a population as a discrete colony of plants separated from other populations by a minimum distance of 1 km. We classified populations into seven size categories (1–25, 26–50, 51–100, 101–200, 201–500, 501–1000 and >1000), according to the number of flowering ramets (shoots with a single inflorescence in flower) in the population. We made an effort to sample populations that were in full bloom and avoided sampling populations at the beginning or end of the flowering season.
Style morph frequencies
To investigate population morph structure (trimorphism, dimorphism or monomorphism), and variation in style morph frequencies among geographical regions and flooding regimes, a total of 73 populations of E. azurea were sampled. In populations with ≤200 flowering ramets, the style morphs of all inflorescences were determined. In larger populations, we sampled at 1·5–2 m intervals along transects positioned 2–3 m apart. In large lakes on the western border of the Pantanal with floating populations, boats were used to enable the sampling of flowering ramets. Because of clonal growth, individual genets could not be distinguished and our estimates of morph frequencies therefore represent phenotypic estimates.
Floral traits
To investigate geographical variation in floral morphology, we measured a total of 24 traits in 34 populations (see Supplementary Data Fig. S1) involving the perianth and sex organs of flowers (see Supplementary Data Table S1) between 2007 and 2012 representing all four flooding regimes. Measurements were made in the field on a single randomly chosen flower per inflorescence using digital callipers (±0·1 mm). Sample sizes ranged from ten to 65 flowers with a mean of 27·6 (±17·24) per population.
To investigate variation in pollen size and production in E. azurea, flower buds were sampled just prior to anthesis in 24 populations representing three of the four flooding regimes (see Supplementary Data Table S1). In the field, flower buds were stored in plastic microtubes with 70 % ethanol. Preliminary studies indicated that there were no significant differences in pollen size and production among anthers within a stamen level of a given morph so that a single randomly chosen anther was used. In the laboratory, one anther per stamen level was removed with forceps and this was mashed in a microcentrifuge tube containing 0·8 mL solution of 70 % ethanol and the surfactant Tween-80. The surfactant assisted in breaking the surface tension. To complete homogenization of pollen grains in the solution, the microcentrifuge tubes were agitated with a vortex (approx. 15–20 s). Using a micropipette, 0·1 mL of the solution was sampled and placed on a haemocytometer to enable estimates of pollen size and number following methods in Lloyd (1965). Pollen size was determined by measurements of the longitudinal axis of five pollen grains per anther level. Sample sizes ranged from two to 46 flowers with a mean of 25·4 (±11·96) per population.
Pollinators
Floral visitors to flowers of E. azurea were sampled in 21 populations representing each of the four flooding regimes in the Pantanal, between December 2011 and September 2012 (see Supplementary Data Table S1). Sampling effort was generally about 3 h per population on fine days when pollinators were active. Insects were killed with ethyl acetate and individually stored and frozen before identification by specialists. For each bee collected, the functional traits proboscides length and body width and length were measured with an optical stereomicroscope and millimetre scaled lens.
Statistical analysis
Style morph frequencies
The mean and overall frequency for each style morph in the 73 populations of E. azurea were calculated. To determine if there were statistical differences in morph frequencies among populations, we used generalized linear mixed models (GLMMs) with binomial errors weighted by the absolute number of individuals per morph in each population, with population nested within flooding regime type as a random effect and style morph as a fixed effect. To test if the morph ratio of each population and the total sample showed a deviation from the expected isoplethic equilibrium (1 : 1 : 1), we used the G-test for goodness-of-fit (Sokal and Rohlf, 2011).
where f (X) = frequency of style morph-X (L = L-morph, M = M-morph, S = S-morph). The index ranges from 0 (monomorphic populations) to 1 (trimorphic population with 1 : 1 : 1 ratios). The morph evenness parameter may be subject to deviations related to sampling effort. To test if there was a relationship between morph evenness and sampling effort, we used Pearson correlation between E and proportional sampling effort, which was the total number of sampled inflorescences in a population divided by the maximum population size (e.g. if a population was classified in the interval of 26–50, we used the value 50). To test the hypothesis that isoplethic populations were proportionally better sampled, a t-test was used comparing the proportional sampling effort between isoplethic and anisoplethic populations, previously determined via G-tests.
Whether style morph evenness was related to population size and flooding regime was tested with generalized additive models for location scale and shape (GAMLSS) using zero-inflated beta distribution (Rigby and Stasinopoulos, 2005; Ospina and Ferrari, 2012). Population size categories were transformed to dummy variables in the model to investigate the shape of the relationship between evenness and population size.
Floral trait variation
To determine patterns of floral variation and their potential causes, the relationship between floral trait variation, flooding regime and pollinator assemblage was explored using GLMMs (Pinheiro and Bates, 2000; Zuur et al., 2009) with a Gaussian distribution and restricted maximum likelihood (REML). Because of differences in the number of populations sampled for floral traits and pollinators (see Supplementary Data Table S1), distinct models were used for each analysis to avoid overparameterization. In the first model, potential factors influencing flower size variation in E. azurea were investigated. The four perianth measurements were summarized using principal components analysis (PCA). To determine the number of axes to represent floral size and shape, we used the Kaiser–Guttman criteria, where only axes with variance >1 are used. Scores of the PCA were then used as the dependent variable, with style morph and flooding regime as fixed effects and population as a random effect.
In a second model, to test if the variation in length of sex organs is related to flood regime, the length of sexual organs was used as the dependent variable, and the type of sex organs [long- (l), mid- (m) and short-level (s) anthers, and long- (L), mid- (M) and short (S) styles] interacting with flood regime as fixed effects, and individuals nested within population as random effects. Because data on pollinators were only obtained from a sub-set of the populations examined in the first model, a third model was used to investigate the relationship between sex-organ variation, flooding regime and pollinator assemblage [represented by the principal co-ordinates analysis (PCoA) axis, see below] and their functional traits (mean values of traits in each population, see below). In this model, pollinator assemblage and functional traits were fixed effects, and individuals nested within populations were random effects. In a final model, variation in pollen grain size and number was evaluated using the different anther levels, flooding regimes and pollinator assemblage and functional traits as fixed effects in the model, and population as a random effect.
The fit of the models was estimated according to residuals and lower values of AICs (Akaike information criteria) and BICs (Bayesian information criteria). We calculated posteriori pairwise comparisons with Tukey specific contrasts (Hothorn et al., 2008). All analyses and graphics used R software (R Core Development Team, 2012), with the packages ‘gamlss’ for the beta-inflated models (Rigby and Stasinopoulos, 2005), ‘reshape’ and ‘plyr’ (Wickham, 2007) for data manipulation, ‘multcomp’ (Hothorn et al., 2008) for specific contrasts, ‘lattice’ (Sarkar, 2008) and ‘ggplot2’ (Wickham, 2009) for graphics, and ‘lme4’ (Bates et al., 2012) and ‘nlme’ (Pinheiro et al., 2012) for generalized mixed models.
Pollinators
The dimensionality of the assemblage of pollinators in each population was reduced using PCoA from an incidence matrix, using the coefficient of Sorensen (see Legendre and Legendre, 1998). Linear regression between the original distances of the matrix (triangular matrix using the Sorensen index) and the final distance achieved via ‘scores’ of the ordination (triangular matrix using the Euclidean index) was used to determine the percentage of recovered variance by the PCoA. From this, we then obtained the number of dimensions to use for representing species composition in the models described above.
The mean pollinator proboscides length, body length and body width of each bee species sampled in each population was determined, and then a grand mean of trait lengths per population was calculated using values for each species. The grand mean can be viewed as a proxy for the degree of pollinator specialization in the population.
RESULTS
Variation in style morph frequencies
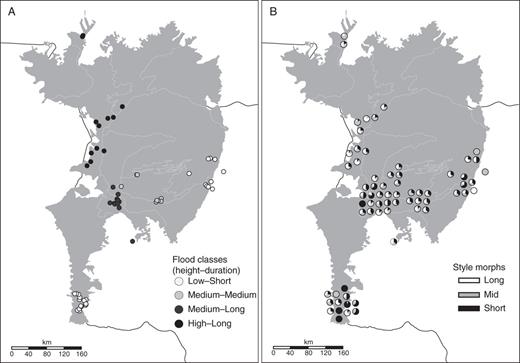
The style morph of inflorescences of 10 008 flowering ramets in 73 populations of E. azurea in the Pantanal was determined. The mean number of ramets sampled per population was 137·1 (s.e. ±2·42; median 66). Twenty-seven populations were in regions with a flood regime of low height and short duration (LS), 16 were in MM, 18 in ML and 12 in HL (Fig. 2A). Seventy-eight per cent of sampled populations were trimorphic (n = 57), with the remaining 22 % equally composed of monomorphic and dimorphic populations (Fig. 2B).
Populations of Eichhornia azurea sampled in the Pantanal wetlands of Brazil. (A) Sampled populations per flood class (see Table 1); (B) pie plots showing floral morph frequency in each population; plots are displaced from their original position to avoid overlap.
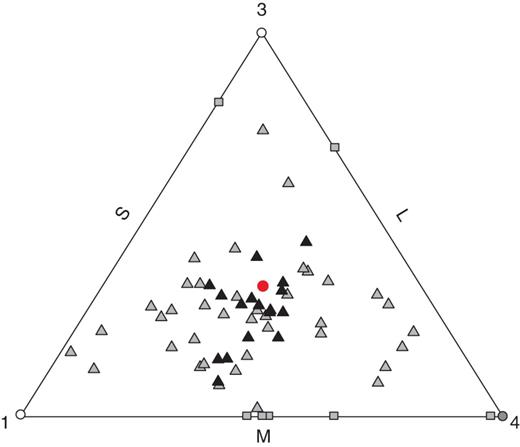
Among the total sample of populations, the overall mean frequencies (± s.e.) of the L-, M- and S-morphs were 0·35 (± 0·025), 0·27 (±0·027) and 0·38 (±0·028), respectively. Style morph ratios exhibited significant deviation from the expected 1 : 1 : 1 isoplethic equilibrium (Gtotal = 19 809·37, d.f. = 146, Gpooled = 135·36, d.f. = 2, both P < 0·0001). Of the 73 populations, 55 were anisoplethic and 18 were isoplethic (Fig. 3; see Supplementary Data Table S2). Proportional sampling effort was similar between isoplethic and anisoplethic populations (t = 1·06, d.f. = 39·09, P = 0·29). Among the 73 populations, the M-morph occurred at a significantly lower frequency than the L- and S-morphs (GLMM binomial model, F = 20·078, d.f. = 3, P < 0·000001; pairwise comparisons S–L, P > 0·05; L–M and S–M, P < 0·001). The same analysis conducted on the 57 trimorphic populations also revealed that the M-morph was less frequent than the other two morphs, but in this analysis the S-morph was less frequent than the L-morph (GLMM binomial model, F = 59·18, d.f. = 3, P < 0·000001; pairwise comparisons S–L, P < 0·014; L–M and S–M, P < 0·001). In 18 of the 57 trimorphic populations, one of the morphs was at a substantially lower frequency (<15 %) compared with the remaining morphs. In 11 of these populations, the minority morph was the M-morph (see Supplementary Data Table S2). Among the eight monomorphic populations, the S-morph occurred in four populations, whereas the M- and L-morphs were found in three and one population, respectively. Six of the eight dimorphic populations were composed of the L- and S-morph with mean frequencies of 0·60 (± 0·08) and 0·37 (± 0·08), respectively.
De Finetti diagram of floral morph frequencies in 73 populations of Eichhornia azurea sampled from the Pantanal wetlands of Brazil. Triangles are populations with the three floral morphs (trimorphic), squares contain two morphs (dimorphic), and circles one morph (monomorphic). The red circle is the equidistant point from all three axes and represents isoplethy. Dots filled with black are isoplethic populations (n = 18) and grey are anisoplethic populations (n = 55) based on G-tests (see the Materials and Methods). Numbers in each vertex correspond to the number of monomorphic populations at that position.
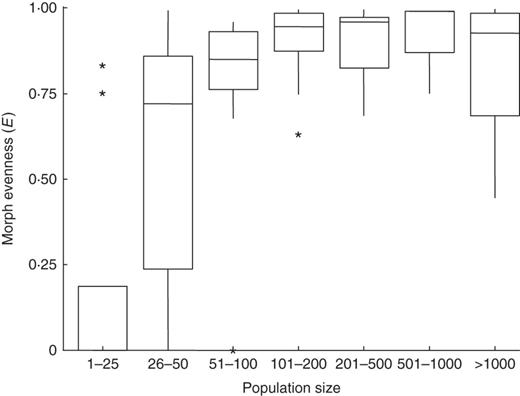
Morph evenness (E) ranged from 0 to 0·997, with a mean of 0·76 (± 0·036), median of 0·887 and coefficient of variation of 0·41. There was a positive relationship between morph evenness and population size (Fig. 4, GAMLSS zero beta-inflated, global deviance = –83·66, AIC = –58·45, BIC = –29·58, estimate = 0·299, s.e. = 0·140, t-value = 2·12, P = 0·037). However, morph evenness was not associated with flood regime (LS, estimate = 0·270, s.e. = 0·567, t-value = 0·476, P = 0·635; MM, estimate = 1·364, s.e. = 0·741, t-value = 1·840, P = 0·07; ML, estimate = 1·179, s.e. = 0·67, t-value = 1·739, P = 0·08; HL, estimate = –0·757, s.e. = 1·019, t-value = –0·743, P = 0·459). There was no correlation between sampling effort and morph evenness (Pearson correlation, r = –0·16, t = –1·3, d.f. = 71, P = 0·18).
Relationship between morph evenness (E) and population size (number of flowering ramets) among 73 populations of Eichhornia azurea in the Pantanal wetlands of Brazil.
Because of earlier reports of semi-homostylous flowers in E. azurea (Barrett, 1978; Alves dos Santos, 2002), a special effort was made during the sampling of style morph frequencies to record their occurrence. Of the 73 sampled populations, 16 had at least one inflorescence with semi-homostylous flowers. The majority of these populations were found in regions with the ML flood regime. In three populations, the number of inflorescences with semi-homostyles flowers ranged between 15 and 20 % (populations 47, 48 and 54; see Supplementary Data Table S2), whereas in other populations this proportion did not exceed 5 %.
Variation in floral traits
The perianth of 778 flowers of E. azurea composed of 284, 241 and 253 L-, M- and S- morphs, respectively, was measured (see Supplementary Data Table S3). Accordingly to Kaiser–Guttman criteria, we only used the first axis of the PCA, which recovered 70 % of the variance and reduced the four perianth measurements to one dimension. The axis showed a strong negative correlation with the four measured traits (flower length, r = –0·905; flower width, r = –0·872; width of the base of the corolla, r = –0·713; width of corolla aperture, r = –0·850). However, we reversed the axis (higher scores correspond to larger flowers) to allow a more intuitive interpretation.
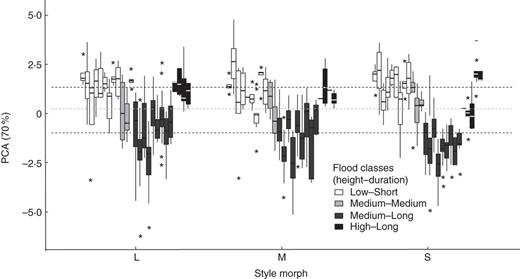
The model using PCA scores to represent perianth size revealed significant differences among the style morphs of E. azurea (Fig. 5; F3, 5200 = 26·905, P < 0·0001); however, when compared using specific contrasts, only flowers of the S- and M-morph were marginally different (estimate = –0·145, s.e. = 0·067, z-value = –22·774, P < 0·076). Overall flower size differed among the flood regions (flood regime; F3, 25 = 19·794, P < 0·0001, see Supplementary Data Table S4), with perianth size significantly smaller in the ML and MM flood regions. There was a significant interaction between style morph and flood regime (F6, 5200 = 2·552, P < 0·018).
Perianth size of flowers of the three style morphs of Eichhornia azurea among flooding regimes in the Pantanal wetlands of Brazil; the values are represented by scores from a principal component analysis and are positively related to flower size. Each boxplot denotes a population and different colours denote the flood classes. Horizontal scattered lines correspond to the grand mean of flower size in each flood region independent of population. Lines for Low–Short and High–Long overlap.
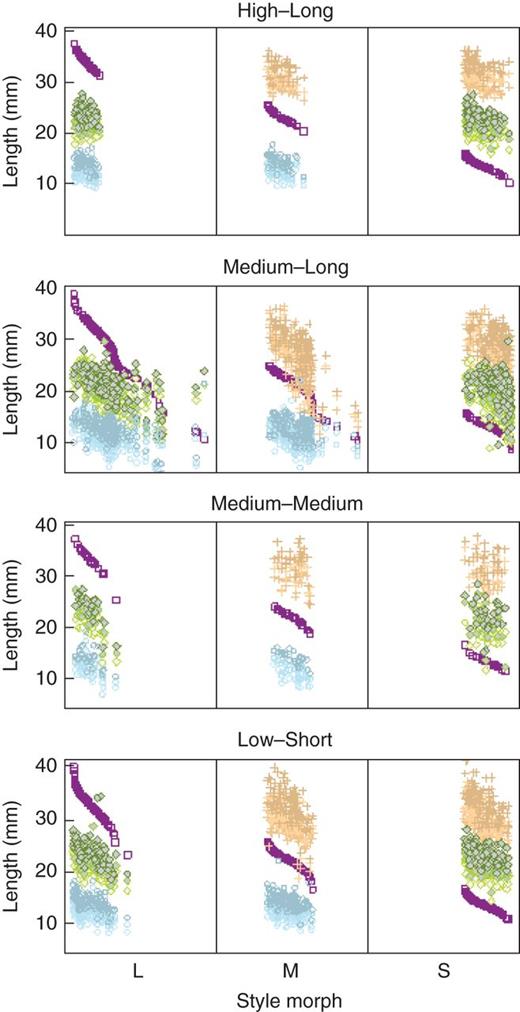
For sex organs, we measured a total of 961 flowers of E. azurea composed of 343, 298 and 320 L-, M- and S- morphs, respectively (Fig. 6; see Supplementary Data Table S5). There was a significant association between the flooding regimes of regions and sex-organ length (F3, 31 = 10·303, P < 0·0001; see Supplementary Data Table S4). The sex organs of populations located in the ML flood regime were shorter compared with the other regions (ML–LS, estimate = –4·322, s.e. = 0·189, z-value = –22·774, P < 0·0001; ML–MM, estimate = –4·372, s.e. = 0·311, z-value = –14·032, P < 0·0001, ML–HL, estimate = –4·440, s.e. = 0·244, z-value = –18·158, P < 0·0001). Pairwise comparisons among the other three flood categories were not significant (see Supplementary Data Table S6) but the interaction between sex-organ types and flood regime was significant (F15, 5739 = 41·242, P < 0·001). In the second model, variation in sex-organ length was partially related to pollinator assemblage (PCoA 1, F1, 406 = 2·060, P < 0·1520; PCoA 2, F1, 406 = 38·222, P < 0·0001; PCoA 1 × PCoA 2, F1, 406 = 1·850, P < 0·174) and was largely explained by the mean length of the proboscides of visitors (F1, 406 = 6·205, P < 0·0131). Pollinator assemblages in ML and HL regions were mainly composed of flies and generalist short-tongued bees, whereas in the LS region they were composed of long-tongued specialist bees, generalist bees and flies.
Variation in stigma and anther height among style morphs in tristylous Eichhornia azurea among the four flood regimes in the Pantanal wetlands of Brazil. Purple squares indicate stigma height, and plus signs indicate the heights of l-level anthers, green diamonds the heights of m-level anthers and blue circles the heights of s-level anthers. Colour gradients within anther height levels represent each of the three anthers per level.
Pollen size and production
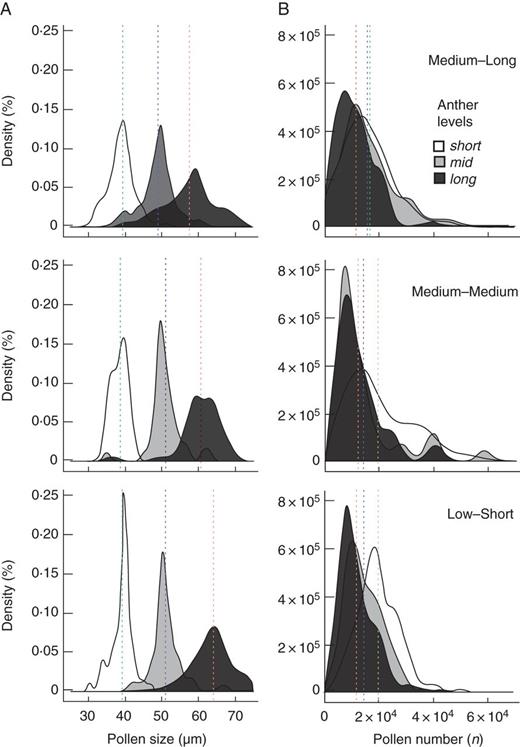
Estimates of pollen size and production were obtained from 611 flowers sampled from 24 populations (n = 219, 199, 193 for the L-, M- and S-morph, respectively). As expected for a tristylous species, the mean size of pollen grains was positively related to anther height (GLMM, F2, 604 = 29 122·381, P < 0·0001; mean pollen size and standard error: long-level anthers of the M- and S-morph = 60·5 ± 0·59 and 61·5 ± 0·48; mid-level anthers of the L- and S morph = 49·8 ± 0·34 and 50·3 ± 0·31; short-level anthers of the L- and M-morph = 38·9 ± 0·22 and 39·4 ± 0·31) (see Supplementary Data Table S6). Overlap of pollen size was more evident between pollen grains from the l- and m-anthers, and more rarely size overlapped between m- and s-anthers (Fig. 7A). Variation in pollen size was significantly associated with flooding regimes (F2,407 = 27·854, P < 0·0001), with pollen from ML populations generally smaller in size than populations from LS and MM regions (ML–MM, estimate = –1·365, s.e. = 0·600, z-value = –2·274, P < 0·0570; ML–LS, estimate = –2·714, s.e. = 0·363, z-value = –7·464, P < 0·001; MM–LS, estimate = –1·349, s.e. = 0·603, z-value = –2·238, P < 0·062; see Supplementary Data Fig. S2). Mean length of proboscides and pollinator assemblage (PCoA 1 and 2) did not explain a significant component of pollen-size variation (proboscides F1, 7 = 0·109, P < 0·751; pollinator assemblage, PCoA 1, F1, 7 = 2·058, P < 0·194, PCoA 2, F1, 7 = 0·006, P < 0·939).
Kernel density distribution of pollen size (A) and pollen number (B) among floral morphs of tristylous Eichhornia azurea among different flood regimes of the Pantanal wetlands of Brazil. Vertical scattered lines are grand means per anther level in each flood category independent of floral morph and population. Red, blue and green lines correspond, respectively, to l-, m- and s-level anthers.
As expected for a tristylous species, pollen production was inversely proportional to anther height (F3,928 = 1127·883, P < 0·0001; Tukey contrast, m–l, estimate = 2694·4, s.e. = 905, z-value = 2·976, P < 0·0082; s–l, estimate = 7973·1, s.e. = 904·9, z-value = 8·811, P < 0·001; s–m, estimate = 5278·7, s.e. = 912·8, z-value = 5·783, P < 0·001; see Supplementary Data Table S6). Pollen production was similar between l-anthers of the M- (mean ± s.e., 11 427 ± 524) and S-morphs (12 010 ± 538), and s-anthers from L- (18 835 ± 642) and M-morphs (17 857 ± 667). However, pollen production was significantly lower in m-anthers of the L-morphs (13 181 ± 500) compared with m-anthers of the S-morphs (17 345 ± 717). Patterns of pollen production did not differ among flooding regimes (Fig. 7B, F2,83 = 1·170, P = 0·3152) nor were they related to the mean length of proboscides and pollinator assemblage of E. azurea populations (proboscides, F1,7 = 1·682, P < 0·235; pollinator, PCoA 1, F1,7 = 0·088, P < 0·774, PCoA 2, F1,7 = 1·792, P < 0·222).
Pollinator sampling
The assemblage of pollinators collected from E. azurea flowers in the Pantanal was composed of 27 morpho-species of bees and flies. The highest diversity occurred in the LS flooding region with 19 species, followed by ML with 13 species and HL with six species (see Supplementary Data Table S7). As pollinators traits were correlated (proboscides × thorax, r = 0·425; proboscides × body, r = 0·819; thorax × body, r = 0·748), and to avoid redundancy and multicollinearity within the models, we used only length of proboscides to represent pollinator functionality. Mean length of proboscides ranged from 1·986 to 22·5 mm, with the highest values in the LS flooding regime (mean weighted by population and s.e., 7·26 ± 8·9) and similar values between the ML (2·66 ± 3·7) and HL (2·69 ± 4·1) regions.
Principal co-ordinates analysis of variation in floral visitors among populations of E. azurea recovered 39·8 % of the variance, reducing the assemblage to two dimensions. The pollinator assemblage differed among flooding regimes (MANCOVA, F4,36 = 3·118, P < 0·026). Populations located in the LS flooding regime were visited mainly by long-tongued specialized bees (Anthophoridae), whereas most pollinators visiting populations in the ML and HL flooding regimes were short-tongued generalist bees (e.g. Trigona sp., Apis mellifera) and syrphid flies (Lycastrirhyncha sp.).
DISCUSSION
Our investigations of the floral biology of E. azurea in the Pantanal wetlands of Brazil revealed three major findings: (1) most populations were tristylous (78 %) and anisoplethic (75 %), with the M-morph significantly lower in frequency than the L- and S-morphs (Fig. 3); (2) as expected, there was a positive relationship between morph evenness and population size (Fig. 4), but, contrary to our predictions, there was no evidence that flooding regimes affected style morph ratios in any systematic way; and (3) measurements of floral traits revealed significant phenotypic differences in mean perianth size (Fig. 5), sex-organ length (Fig. 6), pollen size and pollen production (Fig. 7) among flooding regimes, and there was also evidence that pollinator assemblages varied with the duration of the floods. We also observed a low frequency of semi-homostylous flowers in some populations. We now consider the ecological and reproductive factors that may account for these findings and compare our results with those of studies of floral trait variation in related tristylous species.
Factors influencing variation in style morph frequencies
Clonal propagation is commonly associated with striking deviations from the isoplethic equilibrium predicted for tristylous plant populations. Our surveys of E. azurea revealed that the majority of populations were anisoplethic, and a significant proportion (22 %) lacked style morphs (Fig. 3). Nevertheless, the majority of populations are trimorphic, a finding in striking contrast to earlier surveys of South American populations of the tristylous, clonal relative E. crassipes where stylar monomorphism and dimorphism predominate in the native as well as the introduced range (Barrett, 1977; Barrett and Forno, 1982). This difference in morph structure between closely related Eichhornia species raises the question of which traits facilitate the maintenance of trimorphic populations in E. azurea that are apparently not present in E. crassipes.
Several reproductive features of E. azurea can probably explain the relatively high degree of trimorphism, despite the considerable habitat instability that characterizes the Pantanal. First, small floating colonies and stem fragments of E. azurea were often observed during our survey, and this mechanism of clonal dispersal connects populations by gene flow and may help to maintain trimorphic populations. Secondly, E. azurea is moderately self-incompatible, and disassortative mating among floral morphs is an important process countering the influence of stochastic forces and selfing in disrupting the maintenance of tristyly (see figs 1 and 2 in Eckert and Barrett, 1992). Significantly, unlike E. azurea, E. crassipes is fully self-fertile (Barrett, 1977), and this feature, combined with its free-floating life form, may make it more prone to the influence of founder events following dispersal. Finally, although many aquatic habitats in the Pantanal are ephemeral, E. azurea possesses tough, drought-resistant stems that are able to withstand considerable desiccation. As a result, the persistence of clones over seasons limits opportunities for morph loss from populations. Although clonal propagation in E. azurea is undoubtedly the primary cause of anisoplethic morph structure, our studies provide no evidence that ecological factors in the Pantanal are disrupting the function of tristyly to any significant extent.
Our observations on the frequency of semi-homostylous forms in populations of E. azurea provide another line of evidence supporting the view that the environmental conditions of the Pantanal are largely conducive to the maintenance of tristyly. In all three tristylous species of Eichhornia, the floral polymorphism has broken down, giving rise to semi-homostylous forms (reviewed in Barrett, 1988). However, in each species, selfing forms are characteristic of geographically isolated populations and are usually either fixed in populations, or are the most common form in dimorphic populations [E. azurea, Barrett (1978); Alves dos Santos (2002); E. crassipes, Barrett (1979); E. paniculata, Barrett (1985)]. In contrast, although we observed semi-homostylous forms in 16 of the 73 populations, in the vast majority they represented <5 % of the inflorescences surveyed. Opportunities for the spread and fixation of semi-homostylous forms in E. azurea populations are probably limited by the high concentration of populations in the Pantanal, a lack of geographical isolation owing to the high dispersal of clonal propagules, and sufficient pollinator service to ensure outcrossing.
Our analysis of style morph frequencies in populations of E. azurea revealed that the M-morph was significantly lower in frequency than the L- and S-morphs, a pattern evident in both trimorphic and dimorphic populations. Both stochastic and deterministic forces can cause systematic deficiencies in the frequency of style morphs in populations of tristylous species (reviewed in Barrett, 1993). It is therefore worth considering explanations that might account for the reduced frequency of the M-morph in populations. It is unlikely that stochastic forces operating throughout the Pantanal could account for the reduced frequency of the M-morph (although see Eckert and Barrett, 1995). A systematic bias caused by genetic drift and founder events is more likely to affect the S-morph because of the reduced frequency of the S-allele governing the S-morph in tristylous populations, as discussed further in Heuch (1980) and Barrett (1993). A more likely explanation may be associated with differences in the mating patterns of the style morphs. In contrast to the L- and S-morphs of E. azurea, the M-morph possesses a weak self-incompatibility system allowing high seed set following hand self-pollinations (Bianchi et al., 2000; Cunha and Fischer, 2009). Because the spatial arrangements of sex organs in this morph are more effective at promoting self-pollination, it is possible that the M-morph experiences elevated selfing rates in comparison with the L- and S-morphs (Charlesworth, 1979; Kohn and Barrett, 1992). If this is true, the M-morph may experience strong inbreeding depression and this could drive its frequency down in populations, as has been suggested for Oxalis alpina (Weber et al., 2013). The extensive clonality in E. azurea and the absence of strong self-incompatibility in the M-morph may make this morph particularly susceptible to geitonogamous self-pollination. Measurements in natural populations of morph-specific selfing rates, inbreeding depression and progeny fitness could provide evidence in support of this hypothesis.
Variation in floral traits
Significant phenotypic variation was detected in the floral traits of E. azurea populations occupying different flooding regimes of the Pantanal. These included differences in perianth size, sex-organ dimensions, pollen size and pollen production. In most cases, the majority of populations within a particular flooding regime were in a separate geographic cluster, but populations classified as experiencing a low height and short duration of flooding (LS) were more or less evenly divided between two widely separated geographic clusters. As our measurements were from plants growing in the field, it is not possible to determine the relative contributions of genetic and environmental factors to the observed floral variation.
The most striking pattern of floral differentiation we detected was in populations experiencing a medium flood height for a long duration (ML). Flowers in these populations were smaller with shorter reproductive organs and smaller sized pollen (Figs 5–7). Also, ML populations exhibited significantly greater variance in sex-organ size than the other populations, a pattern suggestive of a reduced intensity of stabilizing selection of stigma and anther position (Fig. 6). Significantly, the majority of semi-homostylous forms that we observed in our survey also occurred in these populations. It is possible that this suite of floral conditions is functionally associated with the pollinator assemblages visiting ML populations. In this region we did not observe the long-tongued bee A. gigas, which is known to specialize on E. azurea and promote cross-pollination (Alves dos Santos and Wittman, 2000), and only generalist bees and flies visited flowers. Thus, the patterns of floral variation in ML populations may reflect relaxed selection on tristylous traits promoting disassortative mating because of inferior pollinator service provided by generalist pollinators. Elsewhere, floral traits of Narcissus species with stylar dimorphism were also more variable in populations lacking long-tongued pollinators (Pérez-Barrales et al., 2014). However, the hypothesis that an absence of specialized long-tongued pollinators may be associated with relaxed selection on floral traits is not supported by data on morph ratios in ML populations, which were not substantially different from other flooding regimes, and also by the fact that flowers in HL populations were among the largest, despite being visited mainly by generalist pollinators. Although little is known about the biology of many of the bees that visit E. azurea, its specialized pollinator, A. gigas, has been found nesting on the ground or in riverbanks (Torchio, 1974; I. Alves dos Santos, Universidade de São Paulo, Brazil, pers. comm.), thus these bees might be disfavoured in the high and long flood regime of the Pantanal with implications for the mating biology and floral traits of populations.
Our measurements of traits comprising the heterostylous syndrome are consistent with what has been observed in related species of the Pontederiaceae with trimorphic self-incompatibility (e.g. Price and Barrett, 1982; Harder and Barrett, 1993; Puentes et al., 2013). Stigma and anther heights are reciprocally positioned in the three style morphs, and pollen size varies with anther height, with the largest pollen produced by long-level anthers, intermediate size pollen by mid-level anthers, and the smallest pollen produced by short-level anthers. These differences in pollen size among anther levels were associated with variation in pollen production, with pollen production inversely proportional to anther height. As expected, no pollen size differences were evident between the same anther levels of different morphs; however, we did uncover a striking difference in pollen production between mid-level anthers of the L- and S-morphs. Mid-level anthers of the S-morph produced, on average, significantly more pollen than the corresponding anther level of the L-morph. This unique pattern appears to be general among species of Pontederiaceae with trimorphic incompatibility, and its developmental basis and mating consequences have been discussed elsewhere (Price and Barrett, 1982; Barrett et al., 1983).
Conclusions
In common with all other studies of style morph frequencies in clonal heterostylous plants, our estimates were based on counts of flowering ramets. Without hypervariable genetic markers (e.g. microsatellites), it would be impossible to estimate the morph ratios of genets. In dioecious plants, differences in the costs of reproduction between the sexes can cause contrasting amounts of clonal growth (reviewed in Delph, 1999); however, there is no empirical evidence that the style morphs of heterostylous species differ in clonal reproduction. Thus, any variation in clone size in populations of E. azurea that we sampled is more likely to result from the residency time of clones in populations and heterogeneity in local resources, rather than intrinsic differences between the style morphs.
The absence of differences in the morph evenness of E. azurea populations among the flooding regimes of the Pantanal was unexpected. In flooding regimes with extended periods of shallower water favourable to sexual reproduction (e.g. LS and MM), we had anticipated that populations would be closer to equilibrium expectations. The similar morph structure and evenness of populations regardless of flooding regime suggest that both sexual reproduction and clonal dispersal are sufficiently common in the Pantanal to erase the signature of founder events from dominating in a region, as is evident in E. crassipes (Barrett and Forno, 1982). The pervasive anisoplethy of morph frequencies that we observed in most local populations of E. azurea is therefore likely to be a non-equilibrium state, with occasional large populations reaching isoplethy.
Depending on the arrival of founding morphs at a site and the subsequent extent of sexual reproduction, progress towards isoplethy in E. azurea populations may vary considerably. In 31·6 % of the trimorphic populations in our survey one of the style morphs was <15 % in frequency. Although the minority morph in a population should experience a frequency-dependent mating advantage causing it to rise in frequency, this may not be realized over relatively short time spans if sexual reproduction is limited by unsuitable conditions for seedling establishment and growth. Although we commonly observed E. azurea seedlings in the Pantanal, the relative importance of sexual recruitment vs. the seasonal dispersal of clones during flooding in promoting trimorphic population structure is unclear. Their relative importance may differ among flooding regimes despite producing similar outcomes with respect to morph frequency variation. Comparative studies of the demography and reproductive ecology of E. azurea in contrasting habitats of the Pantanal wetlands should provide insight into the consequences of reproductive mode for the representation of style morphs in populations.
ACKNOWLEDGEMENTS
We thank M. Delatorre, W. S. Fava and J. Antunes for field assistance in the Pantanal, Isabel Alves dos Santos for bee identification, and Bill Cole for advice on figure preparation. This work was funded by Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia de Mato Grosso do Sul (FUNDECT) (23/200·169/2010 and 23/200·118/2011) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) fellowship support to N.L.C. (BEX4915/13-5), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Research Grant to E.F. (311001/2012-2) and a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to S.C.H.B.